Development of Innovative Research Technique in Genetic Epidemiology of Malaria and Other Parasitic Diseases in Lao PDR for Containment of Their Expanding Endemicity (SATREPS)
Project leader: Dr. Shigeyuki Kano
Member of staff: Moritoshi IWAGAMI,Phonepadith Khattignavong, Pheovaly Soundala, Lavy Lorphachan and other visiting scientists.
Background
Malaria, Schistosomiasis (Schistosoma mekongi) and Opisthorchiasis (Opisthorchis viverrini) have tremendous health burden on the people in Lao PDR. Although significant reductions in malaria transmission have been reported due to the large-scale insecticide-treated bed nets (ITNs) distribution through the Global Fund to Fight AIDS, Tuberculosis and Malaria, strategies based on the scientific evidence have not been developed to deal with the genetic variation in parasites and vectors population, and drug resistant malaria. In fact, artemisinin resistant malaria was reported in Attapeu province in 2014 (Ashley et al., 2014), and it has to be further surveyed in other provinces especially in the Southern part of this country. Estimation of the geographical origin and spread of its resistance is particularly essential for developing control strategies.
Since prevalence of Schistosomiasis (S. mekongi) and Opisthorchiasis (O. viverrini) are localized to Lao PDR and surrounding countries, they are often called as neglected tropical diseases. However, the prevalence of Opisthorchiasis is estimated as high as 15-54% in Lao PDR, causing the unacceptable burden on the people. Indeed, little information on the molecular/genetic epidemiology of the Opisthorchiasis is available to develop effective measures for its prevention.
The Government of Lao PDR requested Japan International Cooperation Agency (JICA) to establish the Lao-Japan Joint laboratory within Institut Pasteur du Laos (IPL) for conducting highly technological research on Plasmodium falciparum and P. vivax, S. mekongi and O. viverrini. The joint research will concentrate on genetic epidemiological studies to detect and control the emergence and dissemination of these parasitic diseases. The Project will also contribute to the capacity development of researchers and technicians in Lao PDR through training of field and Lab work, seminar and career development.
Objective
Objectives of this project are (1) to develop more convenient and accurate methods (PCR methods, LAMP methods, etc.) for diagnosis of the diseases, (2) to monitor temporal and spatial epidemiological situations of pathogens and vectors of the diseases, (3) to analyze mechanisms of emergence and expansion of the drug resistant malaria, especially, artemisinin resistance, and (4) to analyze glucose-6-phosphate dehydrogenase (G6PD) activity of Lao population for evaluation of possible usage of primaquine (Howes et al., 2013), utilizing molecular biological techniques. Based on the scientific evidence obtained by this project, health education for the people will be strengthened and endemicity of the diseases will be monitored together with the local Lao Ministry of Health. Research results will also be utilized in government services for sustainable development of Lao PDR.
Study period of the project
Five years (May 2014 to April 2019)
Study sites of the project
Malaria (P. falciparum, P. vivax):
Savannakhet Province, Attapeu Province, Champasak Province, Saravan Province, Sekong Province, Xieng Khouang Province, Phong Sali Province, Louangphrabang Province
Schistosomiasis (S. mekongi): Khong District, Champasak Province
Opisthorchiasis (O. viverrini):
Khammuane Province, Champasak Province, Saravan Province, Attapeu Province
Study Design, Procedure of Sample Collection and Analyses
Malaria (P. falciparum, P. vivax):
+ Microscopic observation of blood smear, malaria rapid diagnostic tests (RDTs), and G6PD activity assay will be performed using blood samples collected by fingertip prick from the people in malaria endemic areas on sites and/or in the IPL. For G6PD activity assay, G6PD Assay Kit (Dojindo Molecular Technologies, Inc, Japan) will be employed.
+ Blood sample collection on filter papers by fingertip prick from the people in the malaria endemic areas will be performed for DNA analyses, such as PCR methods, LAMP methods, detection of drug (artemisinin, atovaquone, chloroquine, etc.) resistant mutations, and other genetic epidemiological analyses by DNA sequencing (Iwagami et al., 2012 & 2013).
+ Mosquito sample collection in the malaria endemic areas will be performed for identifying species and malaria infection rate by morphological observation and DNA analyses.
+ If possible, in vivo drug (Coartem: artemether-lumefantrine) susceptibility test will be performed at hospitals (or villages) in the endemic provinces.
+ If possible, 2ml of blood will be taken from malaria patients for in vitro drug (Coartem) susceptibility test through Plasmodium falciparum culture and special DNA analysis, i.e. whole genome sequencing by Next Generation Sequencer to identify new responsible drug resistant gene(s) at NCGM laboratory in Japan.
Schistosomiasis (S. mekongi):
+ Microscopic observation of stool sample from the people in Schistosomiasis endemic areas will be performed on sites and/or the IPL. Formalin-detergent method for precipitation of the eggs or Mini Parasep® (APACOR, UK) will be employed.
+ Blood sample collection on filter papers by fingertip prick from the people in the Schistosomiasis endemic areas will be performed for DNA analyses, such as PCR methods, LAMP methods, and other genetic epidemiological analyses by DNA sequencing.
+ Fresh water snail (Neotricula) sample collection in the Schistosomiasis endemic areas will be performed for identifying species of the snail and infection rate of S. mekongi in the snail by morphological observation and DNA analyses.
+ When we find cercaria of S. mekongi in the snail, genetic epidemiological analyses will be performed by DNA sequencing.
+ Microscopic observation of stool sample from Animals (potential reservoirs, such as, Pig, Cow, Water Buffalo, Dog, Cat, etc.) in Schistosomiasis endemic areas will also be performed on sites and/or in the IPL. Formalin-detergent method for precipitation of the eggs or Mini Parasep® (APACOR, UK) will be employed.
Opisthorchiasis (O. viverrini):
+ Microscopic observation of stool sample from the people in Opisthorchiasis endemic areas will be performed on sites and/or in the IPL. Formalin-detergent method for precipitation of the eggs or Mini Parasep® (APACOR, UK) will be employed.
+ Blood sample collection on filter papers by fingertip prick from the people in the Opisthorchiasis endemic areas will be performed for DNA analyses, such as PCR methods, LAMP methods, and other genetic epidemiological analyses by DNA sequencing.
+ Fresh water snail (Bithynia) and Fresh water fish samples collection in the Opisthorchiasis endemic areas will be performed for identifying species of vectors and infection rate of O. viverrini in the vectors by morphological observation and DNA analyses.
+ When we find cercaria or metacercaria of O. viverrini in the vectors, genetic epidemiological analyses will be performed by DNA sequencing.
Ethical clearance
The SATREPS project was approved by the National Ethics Committee for Health Research in the National Institute of Public Health (NIOPH), Ministry of Health, Lao PDR in 2014.
Activities
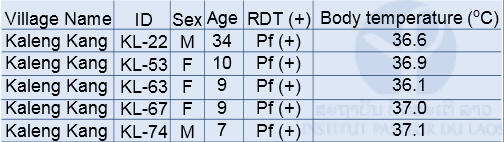
Malaria field survey was carried out in Sepon district, Savannakhet province, in the central part of Lao PDR in August 2014. This field survey was carried out with collaboration with staff from the CMPE, the NIOPH, Malaria Station Savannakhet Provincial Health Department, and a team headed by Professor Kazuhiko Moji in Nagasaki University, Japan. We collected blood samples from people in 3 villages (Kaluk Kao, Kaluk Mai, and Kaleng Kang), Sepon district, for identifying the prevalence and molecular characteristics of malaria. In order to estimate a rate of asymptomatic malaria patient (or malaria carrier), we collected blood samples from both malaria patients and healthy people in the 3 villages (Table 1 & 2). The analysis of the blood samples is ongoing.
RDT: Rapid Diagnostic Test (Malaria Ag Pf/Pv, Standard Diagnostics Inc. Korea)
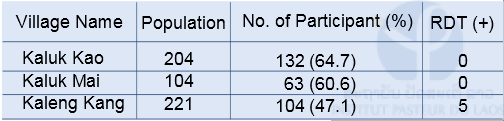
Table 1: Number of participants and malaria positive cases by RDT in the 3 villages in Sepon district, Savannakhet province in 2014
RDT: Rapid Diagnostic Test (Malaria Ag Pf / Pv, Standard Diagnostics Inc. Korea)
Pf: Plasmodium falciparum, Pv: Plasmodium vivax
Blood sample collection
A total of 299 blood samples was collected from people (both malaria patients and healthy people) in 3 malaria endemic villages (Kaluk Kao, Kaluk Mai and Kaleng Kang) on filter papers (FTATM Classic Card, GE Healthcare Life Sciences) by fingertip prick.
DNA extraction from the blood samples (On going):
Total genomic DNA was extracted from all the filter papers using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA) in accordance with the manufacturer’s instruction. Six punched-out circles (2 mm diameter) from the each filter paper were used for DNA extraction. The extracted DNA was eluted with 150 μL of elution buffer in the Kit. The DNA will be used for further analyses such as molecular diagnosis, examination of drug resistant mutation, and population genetic analysis.
Prospective
The Lao-Japan joint Laboratory continues to work in collaboration with the NCGM, the CMPE and the NIOPH, (1) to develop more convenient and accurate methods for diagnosis of malaria, schistosomiasis, and opisthorchiasis, (2) to investigate temporal and spatial epidemiological situations of pathogens and vectors of the diseases, (3) to analyze mechanisms of emergence and dissemination of the drug resistant malaria, and (4) to analyze G6PD activity of Lao population. Further sample collection and DNA analyses of the diseases are ongoing. The SATREPS project will provide the scientific evidence for understanding of the diseases and for the diseases control in Lao PDR and other countries in Greater Mekong Subregion.
Partners
+ Center for Malariology, Parasitology and Entomology (CMPE), Ministry of Health, Vientiane City, Lao PDR
+ NIOPH, Vientiane City, Lao PDR
+ National Center for Global Health and Medicine (NCGM), Tokyo, Japan
+ The University of Tokyo, Tokyo, Japan
+ University of the Ryukyus, Okinawa, Japan
+ Juntendo University School of Medicine, Tokyo, Japan
+ Tokyo Medical and Dental University, Tokyo, Japan
Scientific communications
Oral presentation:
Shigeyuki Kano. Spread of malaria drug resistance in SE Asia. Research Week for Development, Institut francais, Vientiane City, Lao PDR, October 13th-15th, 2014.
Shigeyuki Kano, Moritoshi Iwagami, Bouasy Hongvanthong, Paul Brey. The project for development of innovative research technique in genetic epidemiology of malaria in Lao PDR for containment of its expanding endemicity. The 8th National Health Research Forum, Vientiane City, Lao PDR, October 16th-17th, 2014.
Moritoshi Iwagami, Masami Nakatsum, Daisuke Nonaka, Tiengkham Pongvongsa, Hisami Watanabe, Jun Kobayashi, Futoshi Nishimoto, Kazuhiko Moji, Panom Phongmany, Bouasy Hongvanthong, Paul Brey, Shigeyuki Kano. Genetic epidemiology of drug resistant malaria in Lao PDR. The 8th National Health Research Forum, Vientiane City, Lao PDR, October 16th-17th, 2014.
Poster presentation:
Shigeyuki Kano, Moritoshi Iwagami, Masami Nakatsu, Jun Kobayashi, Daisuke Nonaka, Hisami Watanabe, Tiengkham Pongvongsa, Panom Phongmany, Bouasy Hongvanthong, Paul Brey. Molecular and genetic epidemiology of chloroquine resistant Plasmodium falciparum in Lao PDR under SATREPS project. 13th International Congress of Parasitology, Mexico City, Mexco, August 10th-15ht, 2014.
We wish to thank Dr. Bouasy Hongvanthong, Director of Center for Malariolgy, Parasitology and Entomology (CMPE), Lao PDR, and Project director of SATREPS for his kind support of this project. We thank Dr. Tiengkham Pomgvongsa, Director of Malaria Station, Savannakhet Provincial Health Department, Lao PDR and Professor Kazuhiko Moji, Graduate School of International Health Development, Nagasaki University, Japan for supporting the malaria field survey in Savannakhet. We also thank the staff of the CMPE, the NIOPH, and Sepon Inter-District Hospital, Lao PDR for their contribution to the malaria field survey.
Financial support
This work is supported by SATREPS (Science and Technology Research Partnership for Sustainable Development) from Japan International Cooperation Agency (JICA) and Japan Science and Technology Agency (JST).
References
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. New England Journal of Medicine, 371: 411-423, 2014.
Howes RE, Dewi M, Piel FB, Monteiro WM, Battle KE, Messina JP, et al. Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malaria Journal, 12: 418, 2013.
Iwagami M, Fukumoto M, Hwang SY, Kim SH, Kho WG, Kano S. Population structure and transmission dynamics of Plasmodium vivax in the Republic of Korea based on microsatellite DNA analysis. PLoS Neglected Tropical Diseases, 6: e1592, 2012.
Iwagami M, Hwang SY, Kim SH, Park SJ, Lee GY, Matsumoto-Takahashi EL, Kho WG, Kano S. Microsatellite DNA analysis revealed a drastic genetic change of Plasmodium vivax population in the Republic of Korea during 2002 and 2003. PLoS Neglected Tropical Diseases, 7: e2522, 2013.