Hematophagous insects and ectoparasites in karstic cave areas and their related pathogens in Laos
 Collaboration.
Collaboration.
Lao Army Institute for Disease Prevention
Funding.
Young Research Grant Challenge 2023, Institut Pasteur du Laos Foundation French Ministry of Higher Education and Research (MESRI), France.
Objectives.
The objectives of this project are to: 1) build local capacity on medical entomology and vector-borne diseases; 2) to describe the density and predominant species of cavedwelling insects in two provinces; 3) to screen for the pathogens associated with hematophagous insects and ectoparasites in the karstic cave area.
Background.
Lao PDR is a developing country located in the middle of the Indochinese peninsula and shares borders with many countries where hot spots for biodiversity and potential emerging infectious diseases have been documented. Dengue virus (DENV) and Chikungunya virus (CHIKV) are good examples of emerging diseases. These arboviruses had initially sylvatic transmission cycles where they circulated between vertebrate animals and forest-dwelling insects before they spilled over into the human population. Under specific environmental conditions, some pathogens could cross species barriers and cause the emergence of new zoonotic diseases. Hence, the associated health risks in a specific ecosystem need to be addressed, particularly those related to potential zoonotic pathogens that circulate among karstic and cave-dwelling vertebrates. Very little is known about hematophagous insects and ectoparasites associated with health risks in the Indochinese peninsular as well as in Laos.
From our previous work in 2019, a total of 20,518 sandflies, 1,216 mosquitoes and 95 biting midges were collected from Vientiane province, Laos. The results showed that sandflies and mosquitoes were present with high density. However, samples were not screened for pathogens because of limited funding at that time. Among these hematophagous arthropods, sandflies are well known as vectors for Leishmania parasites as well as Bluetongue virus and other phleboviruses. Mosquitoes are well known as vectors of many flaviviruses such as dengue, Japanese Encephalitis, Zika, and West Nile; and alphavirus such as CHIKV. Culicoides are also suspected as a vector of Leishmania parasites in Southeast Asia. Ticks and chigger mites are vectors of rickettsia and viruses, but there are few studies on these ectoparasites have been done in karstic and cave areas in Laos. Studies on hematophagous arthropods have been neglected in Laos as well as in Southeast Asia countries.
Therefore, to fulfill this gap, we aim to investigate the arboviruses in cave ecosystems, by carrying out arbovirus screening in hematophagous insects and ectoparasites from the karstic and karstic cave areas in Laos.
Methodology.
Study sites The field missions were authorized and facilitated by the Provincial Health Department of two provinces. The hematophagous insects and ectoparasites were collected inside and outside caves alongside karstic limestone mountains (peri-karstic caves) that are located in Vientiane province and Khammouane province, Laos (Fig. 1). One cave from each province was selected.
Figure 1: Study sites, Vientiane and Khammuan provinces
Collection procedures.
The samples were collected during the rainy season, between August and September 2023 for Khammuane and Vientiane provinces. In order to maximize the collection yield and to observe potential temporal patterns, arthropod samples were also collected during the dry season, between November and December 2023. In each selected site, CDC light traps were used for insect collection. The traps were set inside and outside caves, in the forest, and in the village near the cave area from 18:00pm to 6:00 am for 6 nights. Rodent traps also were used for ectoparasite collection from rodents. Ticks were also collected by direct removal from domestic animals by their owners in study areas. Climate data was recorded by Easy data logger.
Specimen preparation and identification.
The samples were stored at −20 °C for 24 hours prior to identification. They then were kept in an Eppendorf tube with silica gel. Mosquitoes and ectoparasites were identified under stereo-microscope using related identification keys 1-6. For the sandflies, specimens were processed and identified at IPL. Sandfly’s head, wings, and abdomen genitalia of both sexes were cut under a stereomicroscope using sterile needles. Head, wings, and genitalia were mounted on slides using a PVA or Euparal mounting medium. Morphological identification was done under a compound microscope using related morphological identification keys 7,8.
Screening for arbovirus detection.
Mosquitoes, sandfly’s thorax and ectoparasites will be screened for phleboviruses, flaviviruses, and alphaviruses by conventional nested RT-PCR 9-11 and Sanger sequencing.
Results.
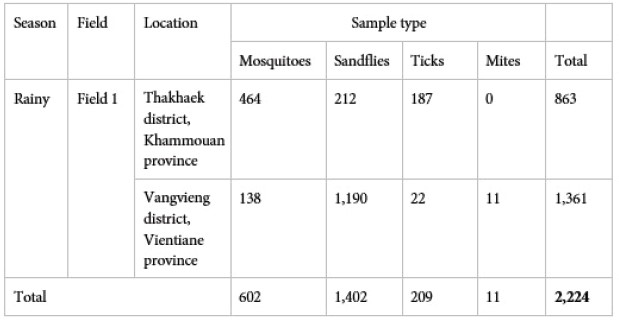
So far, 2 field missions during the rainy season have been completed. A total of 2,224 arthropods were collected, of which 464 and 138 mosquitoes and 212 and 1190 sandflies were collected from Khammouane and Vientiane provinces, respectively. In addition, ectoparasites were collected from domestic animals in each site (Tab. 1).
Table 1: Total number of mosquitoes, sandflies and ectoparasites collected
Discussion
This study aims to detect arboviruses from various cavedwelling blood-sucking arthropods in karstic cave areas in Laos, where the data on the diversity of cavernicolous arthropods of medical or veterinary interests are very limited. So far, a total of 2,224 arthropods have been collected from two provinces and laboratory identification of these arthropods and screening for their pathogens is now ongoing. It is expected that the results from this study will provide a better understanding of cave-dwelling blood-sucking insect vectors in Laos, where the data on the diversity of cavernicolous insects of medical or veterinary interests are very limited.
Conclusion & perspectives
The results of this ongoing project should yield a clearer picture of the risk of exposure to insect vectors in karstic and cave areas in Laos. We will continue our sample collections in the dry season at the end of 2023. We will also continue to screen arthropod samples from all field missions for the presence of arboviruses using RT-PCR and NGS methods.
References
1. Rattanarithikul R, Harrison BA, et al. Illustrated keys to the mosquitoes of Thailand I. Background; geographic distribution; lists of genera, subgenera, and species; and a key to the genera. Southeast Asian J. Trop. Med. public Heal. 36 Suppl 1, 1–80 (2005).
2. Rattanarithikul R, Harbach RE, et al. Illustrated keys to the mosquitoes of Thailand. II. Genera Culex and Lutzia. Southeast Asian J. Trop. Med. Public Health 36 Suppl 2, 1–97 (2005).
3. Rattanarithikul R, Harrison BA, et al. Illustrated keys to the mosquitoes of Thailand III. Genera Aedeomyia, Ficalbia, Mimomyia, Hodgesia, Coquillettidia, Mansonia, and Uranotaenia. Southeast Asian J. Trop. Med. Public Health 37 Suppl 1, 1–85 (2006).
4. Rattanarithikul R, Harrison BA, et al. Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian J. Trop. Med. Public Health 37 Suppl 2, 1–128 (2006).
5. Rattanarithikul R, Harbach RE, et al. Illustrated keys to the mosquitoes of Thailand V. Genera Orthopodomyia, Kimia, Malaya, Topomyia, Tripteroides, and Toxorhynchites. Southeast Asian J. Trop. Med. public Heal. 38 Suppl 2, 1–65 (2007).
6. Rattanarithikul, R. et al. Illustrated keys to the mosquitoes of Thailand. VI. Tribe Aedini. Southeast Asian J. Trop. Med. Public Health 41 Suppl 1, 1–225 (2010).
7. Lewis, D. J. Lewis, D J. 1982. “A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae).” Bulletin of the British Museum (Natural History) Entomology 45, 121–209. Bulletin of the British Museum (Natural History) Entomology vol. 45 121–209.
8. Quate LW. A REVIEW OF THE INDO-CHINESE PHLEBOTOMINAE ( Diptera: Psychodidae ). 4, (1962).
9. Sánchez-Seco, M. P. et al. Detection and identification of Toscana and other phleboviruses by RT-nested-PCR assays with degenerated primers. J. Med. Virol. 71, 140– 149 (2003).
10. Sánchez-Seco, M. P. et al. Generic RT-nested-PCR for detection of flaviviruses using degenerated primers and internal control followed by sequencing for specific identification. J. Virol. Methods 126, 101–109 (2005).
11. Sánchez-Seco MP, Rosario D, Quiroz E, Guzmán G, Tenorio A. A generic nested-RT-PCR followed by sequencing for detection and identification of members of the alphavirus genus. J Virol Methods. 2001 Jun;95(1- 2):153-61.
Publications in 2023
Vongphayloth K, Randrianambinintsoa FJ, Lakeomany K, Phommavanh N, Mekarnia N, Khadri MS, Kaltenbach ML, Huguenin A, Martinet JP, Depaquit J. On the systematics of Phlebotomus Betis and two new related species from Laos with the proposal of the new subgenus Lewisius. Parasite. 2023;30:21. doi: 10.1051/ parasite/2023021. Epub 2023 Jun 9. PMID: 37294211; PMCID: PMC10252460.
Renaux Torres MC, Pellot C, Somwang P, Khositharattanakool P, Vongphayloth K, Randrianambinintsoa FJ, Mathieu B, Siriyasatien P, Gay F, Depaquit J. Phlebotomine sand flies (Diptera, Psychodidae) from Pha Tong cave, Northern Thailand with a description of two new species and taxonomical thoughts about Phlebotomus Stanton. PLoS Negl Trop Dis. 2023 Sep 20;17(9):e0011565. doi: 10.1371/journal. pntd.0011565. PMID: 37729218; PMCID: PMC10558075.






