LHSS COVID-19 Project

Project coordinator: Dr. Vincent LACOSTE and Dr. Somphavanh SOMLOR
Staff members: Dr. Thonglakhone XAYBOUNSOU, Phaithong BOUNMANY, Sitsana KEOSENHOM, Souksakhone VIENGPHOUTHONG, Somsanith CHONEPHETSARATH, Kitphithak FANGKHAM
Trainees: Kedkeo INTAVONG and Longthor
Funding: LHSS under USAID, Luxembourg and French Embassy
Background
Soon after the emergence of the Severe Acute Respiratory Syndrome – Coronavirus 2 (SARS-CoV2) virus in the city of Wuhan, China at the end of 2019, which led to the COVID-19 (Coronavirus Disease 19) pandemic, the Institut Pasteur du Laos (IPL) was requested by the Ministry of Health (MOH) to participate in the national response to the pandemic. Since then, IPL has been one of the frontline laboratories for the diagnosis of SARSCoV2 in the country.
To strengthen diagnostic and training capacities in Laos as part of the COVID-19 response, LHSS initiated the Lao Pasteur COVID-19 activity, which started on October 1, 2020. LHSS engaged IPL as a subcontractor to perform the key tasks in this activity. The activity had three main objectives:
• Perform the molecular diagnosis of SARS-CoV2 using RT-PCR testing
• Generate SARS-CoV2 sequences to identify variants
• Train laboratory technicians on COVID19 diagnosis
RT-PCR TESTING OF SARS-COV2
Objectives and overview
The main objective of the diagnostic testing was for LHSS to improve the government’s diagnostic capacity for the detection of SARS-CoV2. LHSS, through its subcontractor IPL, aimed to increase the number of samples tested by RT-PCR within the country through systematic testing of a portion of travelers entering the country, suspected local cases, and close contacts, with a target of approximately 13,250 tests by the end of September. Samples were mostly nasopharyngeal, though at times naso + oropharyngeal.
Results
Initially, IPL screened samples obtained from World Food Program flights. However, these flights were infrequent – a maximum of one per week – and IPL did not obtain sufficient samples to meet expected activity targets. Therefore, to increase the volume of activity, IPL reached an agreement with the director of the National Center for Laboratory and Epidemiology (NCLE) to also provide testing for passengers arriving at the Wattai International Airport, Vientiane, on two flights per week from Kunming, China. This allowed IPL to quickly achieve and exceed the goals set forth in the work plan of the project (Figure 3). In fact, this enabled IPL to make up for the delay accumulated (in terms of a number of tests) during the first two months of the project and to reach (and subsequently exceed) the expected numbers during week 10 (Figure 4).
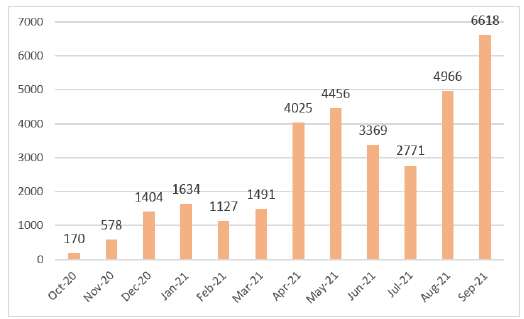
Figure 3. Monthly SARS-CoV2 RT-PCR diagnostic
activity
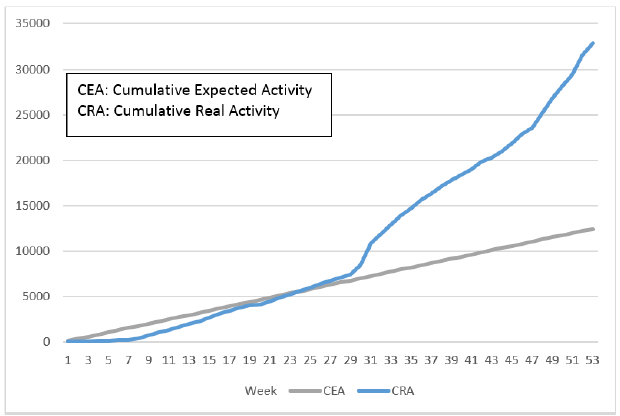
Figure 4. Evolution of the diagnostic activity (RTqPCR SARS-CoV2) per week (week 1 corresponding to the start of the project, i.e. the week of October 1). In blue is the expected volume of activity, in blue is the actual volume of activity.
In early April 2021, more than a year after the start of the pandemic, IPL had identified only one positive case (from April 2020). As of September 2021, IPL had identified a total of 340 positive cases out of the 32,458 tests carried out since the start of the LHSS activity, which began on October 1, 2020.
RT-PCR as part of EPI-Surveillance
All RT-PCR results were shared with NCLE and DCDC on a daily basis; Excel files with test results were also shared each quarter with USAID. NCLE consolidated testing data from all laboratories and reported aggregate data to the MOH. This active surveillance enabled the MOH to track the epidemiologic situation, providing information to track close contacts to limit the spread of the virus. In addition, this information was used for public health decision-making.
Key challenges and lessons learned
With the entry of the virus into the country in mid- April 2021 at the time of Pi Mai Lao, followed by the entry of the Delta variant in mid-June, our diagnostic activity increased dramatically during the latter half of the activity – with more than 3,900 tests on average per month since April. By the end of August, IPL had carried out a total of 32,468 diagnostic tests, thus doubling the number of tests expected by the end of the activity. To deal with this huge increase in activity, IPL had to adapt to handle such a large volume of samples on a given day. In addition, to reinforce the team, IPL engaged other institute staff in COVID-19 diagnostics. This significant increase in activity had consequences on the management of reagents and consumables and therefore on the dedicated budget, requiring IPL to obtain supplemental funding for reagents and consumables. The large volume also had consequences for the supply and delivery time of these reagents and consumables as stocks were running out around the world. Though we were able to obtain the needed products in time for testing, the large volume necessitated more frequent and larger volume orders.
Covid-19 sequencing
Objectives and overview
The main objective of the genomic sequencing task was to perform the sequencing of positive cases to follow the evolution of the SARS-CoV2 strains and identify the entry and spread of variants. IPL signed a material transfer agreement with NCLE to obtain SARS-CoV2 RT-PCR positive samples from them and to perform genomic sequencing on specific portions of the genome. IPL also conducted sequencing on some positive samples identified at IPL.
Results
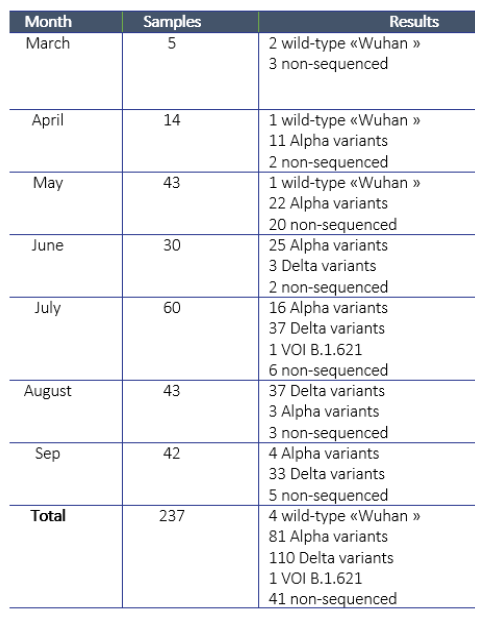
IPL received the first samples for sequencing from NCLE in March 2021. Since then, IPL attempted to sequence a total of 237 samples and obtained sequences for a total of 196 samples. For the remaining 41 samples, the laboratory was unable to obtain an amplification product, due to a too low viral load (Ct> 30). IPL investigated alternative approaches for sequencing and procured equipment to conduct next-generation sequencing (which works even with lower viral loads), but did not obtain all equipment in time for this activity.
Regarding the 196 sequences obtained, four cases were infected with the “wild” strain of Wuhan in March, April, and May at the origin of the pandemic, 81 were infected with the Alpha variant (English variant), 110 by the Delta variant (Indian variant), and finally, one by a variant of interest, VOI B.1.621, initially identified in Colombia (Table 1). The Alpha variant is the one that entered at the turn of the Lao New Year in April 2021, which then spread throughout the country before being replaced by the Delta variant detected in June and responsible for the huge epidemic wave observed since then.
Table 1. Sequencing activities and results.
Genomic sequencing as part of epi-surveillance All sequencing results were shared with the NCLE, WHO and all partner laboratories immediately, by email first and then via a database accessible virtually by various partners. On August 20, IPL submitted “prototypic” sequences (18 sequences) representative of the various variants identified in Laos on the GISAID database (https://www.gisaid.org/). Sequencing results allowed the identification of the circulating strains present in Laos. These results have important implications for the MOH, to support their efforts to track the spread and to reduce the COVID-19 burden. This is in part because different variants present the different potentials for spreading, and can inform the establishment of different mitigation strategies depending on the situation (opening of quarantine centers, closing schools, restaurants, borders, restrictions of movements between provinces, districts, etc). Thus, the identification of variants was invaluable to the COVID-19 response in Laos.
Key challenges and lessons learned
Lao PDR has a very limited capacity for genomic sequencing. Indeed, only the Institut Pasteur du Laos is able to do this type of analysis, with only a few people trained to analyze and interpret the sequence results. The sequencing results generated at IPL provided important data to the MOH which was used to decide which strategies to apply to limit the spread of COVID-19 in the country.
The sequencing capabilities acquired during this activity by certain technicians and scientists at IPL have strengthened their technical capacity and can be useful in the analysis of other pathogens. This could be useful in the future to rapidly respond to new emerging or reemerging diseases.
Lastly, to circumvent the problem of sequencing samples for which the viral load was low (Ct> 30), IPL pursued an alternative – the MinION technology from the Oxford Nanopore Technologies company, which makes it possible to generate sequences even from low viral load samples. Unfortunately, the reagents and consumables were received only in late July and therefore were not used for the sequencing conducted for this activity.
Training objective & approach
The overarching objective of the activity and of the training was for LHSS to enhance the government’s diagnostic capacity for the detection of SARS-CoV2. LHSS, through the subcontractor IPL, aimed to increase the number of samples tested by RT-PCR by training local laboratory technicians in the necessary diagnostic techniques. The goal of the training performed by IPL was to train four laboratory technicians in bio-safety, bio-security, extraction, reagent preparation, RT-PCR techniques, and all steps of COVID-19 diagnostics (which are also applicable for the other viral diseases). The training was initially designed to last for six months, with some initial theoretical training followed by hands-on practical training. Due to challenges faced in recruiting laboratory technicians, the initial two trainees stayed on for the entire year, and therefore the training ultimately involved an initial 3 months of theoretical training followed by 9 months of practical training.
Selection of trainees
In selecting the initial two candidates for training, IPL consulted with the faculty of Medical Technology at the University of Health Sciences to consider expectations for the training and possible candidates. A list of interested students was provided to IPL by a professor at the University. IPL clearly explained to the candidates the criteria for selection, which would involve a fair and non-biased assessment to identify the best candidates. IPL administered a brief oral interview and written test to all interested candidates; the top two performers (in terms of test scores as well as suitability for the training) were selected for training.
End of training skills assessment
When the formal training concluded, the two trainees were assessed on their theoretical understanding with a post-test. The two trainees obtained post-test scores of 54/60 and 58/60 (see Annex II). Both trainees followed the training closely and participated actively in teamwork. At the end of the training, they were able to perform RTPCR independently, work in BSL3 and even analyze the sequence results. Their skills were assessed in an end of- training skills assessment, in which both trainees demonstrated their mastery of the necessary skills. When the need arose for training laboratory technicians in the military, the two IPL laboratory technicians assisted in training these technicians on RT-PCR, therefore further strengthening local capacity.
Challenges and lessons learned
With the COVID-19 lockdown and Universities shutting their doors, IPL was unable to identify two additional qualified candidates to recruit for the second round of training. Therefore, instead of four, IPL was able to train only two trainees. However, these two trainees provided much-needed support to IPL in conducting a large number of tests, especially when cases skyrocketed in April 2021 and IPL was faced with conducting dozens of tests per day. Therefore, the trainees were able to support IPL in conducting additional tests to support the government’s diagnostic capacity. The training was also an ideal opportunity for the trainees to apply their new skills on the job as they applied key concepts and also learned how to transfer what they learned to others.
Trainees were selected in part based on their interest and career goals, to ensure that they were suitable for the training and intended to continue their work on the implementation of RT-PCR to assist the country in the response to COVID-19 and other viral outbreaks in the future. This training was very effective in increasing the capacity of human resources as participants improved their leadership skills and confidence in working with dangerous viral diseases, as well as their ability to explain and share their knowledge with others and work well with the team and medical staff in the hospital network. In fact, both trainees have also provided support to train military laboratory technicians in performing RT-PCR.
Recommendations
Based on experience from this activity, LHSS and IPL share the following recommendations for the GOL to consider:
• Improve coordination between the reference laboratories and the provincial laboratories for a full standardization of the pre-analytic steps: a collection of samples, storage of samples, packing and transportation of biological hazardous samples
• Train/sensitize medical staff on different aspects of sample collection, transport and analysis so that they are aware of the procedures and can provide the necessary support
• Apply standard operating procedures in all partner labs, to have comparative results
• Coordinate between the reference laboratories and the provincial laboratories in the selection of samples for the performance of genomic sequencing, so that the sampling is representative of diverse geographical areas
• Share feedback on the quality of samples with partner laboratories who are sharing samples, so that they can address any issues with sample quality accordingly
• Train additional laboratory technicians in the provinces as well as students at the university level in conducting RT-PCR
• Provide practical training on RT-PCR to all laboratory technology students
• Continue to build the capacity of other laboratory technicians for current and future health emergencies – by developing their capacity in molecular biology techniques (from the extraction of nucleic acids to amplification for detection and sequence analysis) LHSS and IPL also suggest some longer-term strategies to strengthen future responses to SARS-CoV2 and also other pathogens: • In the molecular biology curriculum at the University, establish a course with theoretical and practical sessions to train a new generation of technicians and scientists in PCR, sequence analysis and phylogeny — from the sample collection to the interpretation of results.
• Establish a central laboratory where samples can be sent by partners for sequences to be generated. This lab should have different types of equipment for Sanger and next-generation sequencing, for public health activities (COVID-19 and other pathogens), where every suspect sample can be tested and sequenced.
Conclusion
The implementation of this activity has enabled LHSS and IPL to make a significant contribution to the national effort to combat the COVID-19 pandemic by performing RT-PCR tests and to COVID-19 response strategies by generating viral sequences that allowed for the identification of variants. The project has also helped IPL to introduce new technologies to the country (next-generation sequencing), which will be used in future activities.
- Train the trainee on sample collection.
- The trainee helps the team to analyse sequence results
- The trainee passes on the knowledge to the military trainee.
Publication
1. Calvez E, Vetsaphong P, Somlor S, et al. First probable case of congenital Zika syndrome in Lao People’s Democratic Republic. Int J Infect Dis. 2021;105:595-597. doi:10.1016/j.ijid.2021.03.019