Malaria vectors in Lao People’s Democratic Republic and Thailand and capacity building in medical entomology (MALVEC)
Project leader: Dr Sebastien Marcombe and Dr Paul Brey
Member of staff: Julie BOBICHON (Junior scientist), Boutsady SOMPHONG (Technician) and Nothasine PHOMMAVANH (Technician)
Background
In Lao PDR, a recent national survey on the distribution of malaria showed that 65% of the population was still living in transmission areas (Jorgensen et al., 2010). This study also showed the predominance of Plasmodium falciparum particularly in the southern part of the country associated with a high risk of transmission.
In 2004, an entomological survey showed that Anopheles dirus was an important malaria vector despite its low density and that the role of An. minimus in the transmission varied over time and space (Trung et al., 2004). However, the successive appearance in tropical forest areas of An. minimus during the dry season and An. dirus s.s. during the second part of the rainy season allows a sustainable malaria transmission. More worrying, the recent environmental modifications linked to agriculture and forestry culture (e.g. rubber plantations) may change the status of several vectors, secondary and major, by giving them appropriate ecological conditions to thrive (Osbomer et al., 2007). Insecticide bioassays showed that An. minimus was resistant to pyrethroids in northern Vietnam and Thailand and An. epiroticus was resistant to DDT and pyrethroids in Cambodia and southern Vietnam (Van Bortel et al., 2008). It is possible that the use of agricultural insecticides may be at the origin of the selection of these resistances and so constituting a danger for the implementation of effective vector control strategies. Unfortunately, there is a paucity of data available on the insecticide resistance of the main malaria vectors in Lao PDR. The “hot-spots” of transmission being located in border zones (Thailand, Cambodia, Vietnam…), there is an important risk of dispersal of the population of vectors and the resistances in the surrounding areas. In Lao PDR no data are available regarding the impact of agriculture pesticides on the resistance selection. The only available means of control of the transmission is the use of pyrethroid treated bed-nets, but in Laos, 30 to 50% of the people at risk sleep under treated bed-nets. We do not know if the malaria vectors from Thailand and Lao PDR are endophagic or exophagic. For example, An. dirus is known to be exophagic, biting people at twilight at a time of day when that is not protected by treated bed-nets. Hence, it is necessary to understand the vectors biology in Lao PDR and Thailand to adapt the vector control strategies.
The risk of distribution of the insecticide resistances of vectors in South-East Asia represents a serious threat to the good results recorded these last years in the control of malaria. It is urgent to identify the distribution, the levels and the mechanisms of resistance of the vectors in the lower Mekong countries with the aim of helping the health authorities to develop more effective strategies of prevention and control of the disease.
Objectives and outcomes
This project has 4 fundamental objectives:
+ Evaluation of vectors bionomics and distribution and their role in malaria transmission
+ Evaluation of the levels, types and mechanisms of insecticide resistance
+ Evaluation of the impact of environmental factors on vector dynamic and resistance selection
+ Capacity building in medical entomology in Lao PDR
Expected outcomes:
+ Set up a comprehensive map representing the “hot spots” for malaria transmission in Lao PDR and Thailand (border area)
+ Generate an Insecticide Resistance database in the main malaria vectors
+ Address the dynamics and gene flows between malaria vectors populations
+ Guide public health authorities in the design and implementation of Insecticide Resistant Management strategies
+Capacity strengthening of Lao and Thai students in medical entomology and vector control
Methods
Field sites
The study took place in 10 provinces distributed throughout Lao PDR and 5 districts in Ubon Ratchathani province in Thailand (Figure 1). This enables to have a global vision of the situation in the country. CMPE already have an important collaboration network with all the health district departments, which is a fundamental parameter for the success of the project.
- Figure 1. Sampling locations in Lao PDR
Mosquito collection
Every village was divided into four zones from a central axis to select at random 1 house by zone. The selected houses were distant from each others of at least 30 meters. For every house a collector was placed inside and another one outside from 6:00 pm in the evening till 6:00 am in the morning on 4 consecutive nights. Mosquitoes were collected with glass tubes (Figure 2). Mosquitoes were also collected overnight with the cow/buffalo bait collection method. A long mosquito net was disposed around the animal and adult mosquitoes were collected on it (Figure 3).
- Figure 2. Human landing catching
- Figure 3. Buffalo bait collection
Mosquito identification
The mosquitoes collected were morphologically identified to the species or complex using microscopes and following the identification keys (Medical Important Anophelines of Southeast Asia) (Figure 4). The Anopheles collected were then separated by species for the insecticide resistance tests.
Insecticide resistance
Insecticide bioassay (tube tests) were performed following WHO protocols to measure the insecticide susceptibility of the different mosquito species collected (WHO 2013). Adult female were exposed to DDT (4%), deltamethrin (0.05%), and permethrin (0.75%), the main insecticides used in public health in Lao PDR.
- Figure 4. Field laboratory in Vientiane province
- Figure 5. Insecticide resistance test.
Results 2015
Mosquito abundance and diversity
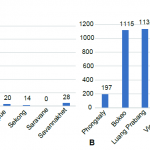
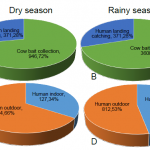
Figure 6 shows the number of Anopheles collected in the 10 provinces during the dry and rainy season. More mosquitoes were collected during the rainy season and Luang Prabang, Vientiane and Bokeo province showed the highest number of Anopheles sp. collected. Figure 7 (A and B) shows the number of Anopheles sp. collected on human and cow during the dry and wet season 2015. During the dry season, a total of 1317 Anopheles sp. was collected and among them 28% were collected on human. During the rainy season, 5150 Anopheles sp. were collected and among them 30% on human. Figure 7 (C and D) shows the Anopheles sp. collected from human landing catching. During the dry season, 34% were collected indoor and during the rainy season 47% were collected indoor.
- Figure 6. Total number of Anopheles sp. collected from Human Landing Catching in the ten provinces during dry and rainy season 2015 (A and B).
- Figure 7. Total number of Anopheles spp. collected from human landing catching and cow bait collection during the dry and rainy season 2015 (A & B); inside and outside the house (C & D).
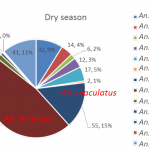
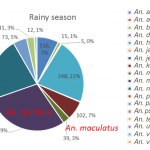
During the dry season, 13 different species of Anopheles sp. were collected on human (Figure 8). The two most abundant species were Anopheles minimus, An. maculatus which are the primary malaria vectors in the Lao PDR (primary vectors, n=55 and n=170 respectively). During the rainy season, 18 different species of Anopheles sp. were collected on human (Figure 9). The most abundant species was An. minimus, representing 29% of the total of mosquito collected on human (n-453).
- Figure 8. Abundance and proportion of Anopheles spp. collected from HLC during the dry season 2015.
- Figure 9. Abundance and proportion of Anopheles spp. collected from HLC during the rainy season 2015, Lao PDR.
Human biting rates
Figure 10 shows the human biting rates (number of Anopheles collected per man per night) in the 10 provinces in 2015. During both dry and wet season Vientiane and Luang Prabang provinces collection presented the highest HBR. HBRs were lower in average during the dry season compared to the rainy season.
- Figure 10. Human biting rates during the rainy (A) and dry (B) season 2015 in the 10 provinces. HBR is the number Anopheles sp. collected per human per night.
Insecticide resistance
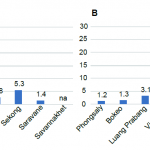
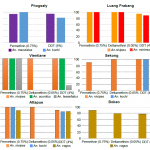
The results are shown figure 11. Several species showed resistance or a reduced susceptibility to the insecticide tested. Among the primary vector tested, Anopheles maculatus showed a reduced susceptibility to permethrin and DDT in Phongsaly while An. minimus also showed a reduced susceptibility to DDT in Luang Prabang. The secondary malaria vector An. nivipes showed resistance to the 3 insecticides tested.
- Figure 11. Resistance status of Anopheles sp. against permethrin, deltamethrin and DDT, Lao PDR 2015.
Discussion and perspectives
The results from Lao PDR showed that An. minimus and An. maculatus, primary vectors of malaria, and several secondary vectors, are biting humans constantly during the night both indoors and outdoors. This emphasizes the need for use of bed nets when people are sleeping and personal protection when people are outside. However insecticide resistance tests showed that several Anopheles species are resistant to DDT and to pyrethroids (used for bed nets coating) in several provinces of the Lao PDR, emphasizing the need for a constant monitoring of insecticide resistance in malaria vectors in the area.
Molecular work to identify the sibling species of the different group/complex of Anopheles species started in 2015. Plasmodium detection in these mosquitoes will also be implemented. Furthermore the possible mechanism involved in insecticide resistance, metabolic and target mutation, will be researched.
Partners
+ National Center of Malariology, Parasitology and Entomology (CMPE), Vientiane, Lao PDR
+ Institut de Recherche pour le Développement (IRD) IRD-MIVEGEC, IRD UMR-MD3, Bangkok Thailand
+ Kasetsart University, Department of Entomology, Bangkok, Thailand
+ Institut de Médecine Tropicale d’Anvers (IMTA), Belgium
+ University of Life Sciences (ULS), Oslo, Norway
+ Bureau of Vector Born Diseases (BVBD), Ministry of Health, Thailand
+ World Health Organization (WHO)
Financial support
The MALVEC project was initiated thanks to the research grant from the Initiative 5% of the Global Funds to fight Aids, Tuberculosis and Malaria and Expertise France
References
Jorgensen P, Nambanya S, Gopinath D, et al.: High heterogeneity in Plasmodium falciparum risk illustrates the need for detailed mapping to guide resource allocation: a new malaria risk map of the Lao People’s Democratic Republic. Malar J 2010, 9(1):59.
Trung HD, Van Bortel W, Sochantha T, et al.: Malaria transmission and major malaria vectors in different geographical areas of Southeast Asia. Trop Med Int Health 2004, 9(2):230-237.
Van Bortel W, Trung HD, Thuan le K, et al: The insecticide resistance status of malaria vectors in the Mekong region. Malar J 2008, 7:102.