Vector mapping, characterization of insecticide resistance of Aedes populations, and entomology capacity development in Lao PDR
Project leader: Dr. Sebastien Marcombe, Dr. Ian Sutherland and Dr. Paul Brey
Member of staff: Khamsing Vongpayloth (Scientist) Noy Khounsombat, (Laboratory technician) Nothasine Phommavan (Laboratory technician)
Bounma Xayasouk (Mosquito insectary technician)

Background
Because of global changes (environment, climate) and increasing transportation, in the past decades we have seen a dramatic resurgence of dengue and chikungunya throughout regions where Aedes mosquito vectors are present and this has led to major public health problems (WHO 2006, Benedict et al., 2007). In 2013, Lao PDR faced one its most severe dengue outbreak in years (Figure 1). The mosquito Aedes aegypti is the main dengue virus vector in Lao PDR (Dr Vongphayloth pers com.). Another species, Aedes albopictus, is a secondary vector of dengue, but is also the vector of the chikungunya virus that is reemerging in Southeast Asia and in Lao PDR. Because there is still no vaccine or specific treatment available against these viruses, vector control remains the only strategy for reducing dengue or chikungunya transmission. Effective vector control measures rely on active community participation, health education programs, and environmental management that include improvement of water supplies and storage, solid waste management, and modification of human-made larval habitats (Erlanger et al., 2008). During inter-epidemic periods or when the elimination of breeding habitats of the mosquito is not easily achievable, insecticide application in larval habitats is routinely conducted by public health services in many countries including Lao PDR (Thavara et al., 2004).
Space spraying applications are conducted during epidemics or when the entomological indices of mosquitoes are high. For both larviciding and adulticiding, organophosphates and pyrethroids are the insecticide families of choice worldwide as well as in Lao PDR.
Unfortunately, many dengue vector control programs are threatened by the development of insecticide resistance in Aedes populations across the world. Insecticide resistance is associated with mutations in the sequence of the target protein that induce insensitivity to the insecticide (target-site resistance, knock-down resistance mutation, kdr), and/or the up-regulation of detoxification enzymes (metabolic-based resistance, P450 monooxygenases (P450s), glutathione S-transferases (GSTs) and carboxy/cholinesterases (CCEs)).
Strong levels of resistance to organophosphates and pyrethroids have been detected in Aedes aegypti population in Southeast Asia (Ranson, 2010). Resistance to these same families of insecticide has also been detected in Aedes albopictus populations worldwide (Kamgang et al 2010, Marcombe et al. 2014). The organophosphate temephos (larvicide) and insecticides from the pyrethroid family (permethrin, deltamethrin; adulticides) have been used in Lao PDR for decades to reduce the vector populations during important dengue epidemics but to date, compared to its neighboring countries there is no information available on the resistance status of Aedes populations and the possible impact of the resistance on vector control operations in the country.
The risk of insecticide resistance in dengue vectors in South-East Asia represents a serious threat to the achievements seen in dengue control during recent years. It is urgent to identify the distribution, the levels, the mechanisms, and potential environmental factors of resistance in dengue vectors in the lower Mekong countries to assist health authorities to develop more effective strategies of prevention and control of the disease.
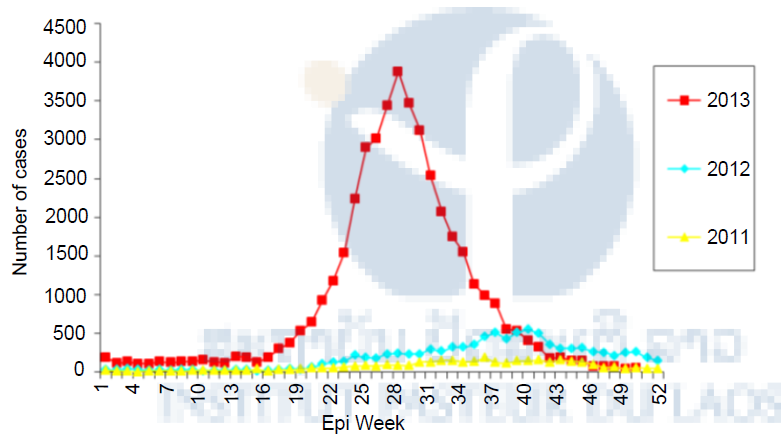
Figure 1. Dengue cases in Lao PDR, 2011 – 2013. Data from Source: http://www.wpro.who.int/emerging_diseases/documents/dengue.updates.2013/en/
Objectives and expected outcomes
The purpose of this project is to provide entomology capacity building, identify circulating levels of insecticide resistance (IR), and improve data on vector risk profiles in high priority location in Lao PDR.
Objectives:
+ Evaluation of the levels of insecticide resistance in the vector populations in Lao PDR.
+ Evaluation of the types and mechanisms of insecticide resistance (i.e. metabolic or target site) in Laos.
+ Evaluation in semi-field trials of common insecticide formulations used in Lao PDR versus candidate insecticides for larval control Capacity building in medical entomology in Lao PDR.
Expected outcomes:
+ Set up a comprehensive map of the levels of insecticide resistance in Lao PDR
+ Generate an Insecticide Resistance database in the main dengue vectors in Lao PDR
+ Guide public health authorities of Laos in the design and implementation of Insecticide Resistant Management strategies.
+ Capacity strengthening in medical entomology and vector control in Lao PDR.
Methods and Results
Mosquito collections:
During the rainy season of 2014, collections were made in Vientiane capital, Xayabury and Luang Prabang provinces and larvae collected by collaborators from Saravane and Attapeu (CMPE and district staff) were sent to the Institut Pasteur. In Xayabury province, larvae were collected in breeding habitats in several villages and brought back to the laboratory for rearing. Larvae were collected in the forest surrounding Tinkheo village and in rubber plantations (rural areas) in Luang Prabang province. Larvae were also collected in Luang Prabang city in households and in temples (urban area). Sentinel sites were established in Vientiane Capital in several districts and villages and larval collections were made every week at these locations. All the collection sites were geo-referenced (Figure 2 & Table 1). Another collection was made in Luang Prabang in July 2015 to collect Ae. albopictus populations. (Figure 3).
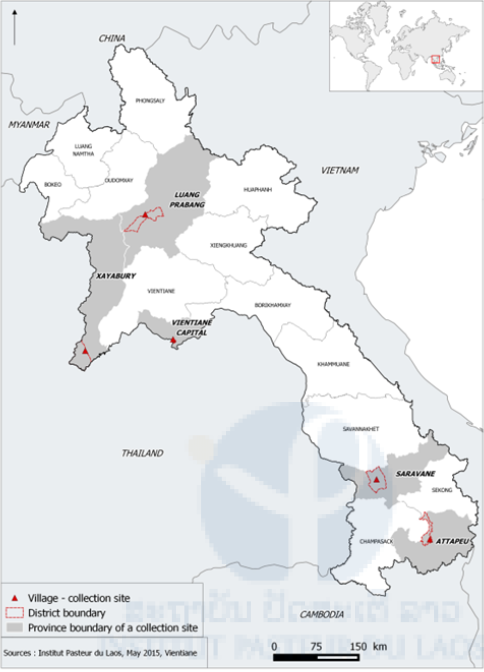
Figure 2. Location of the mosquito collection sites.
Table 1. List of the Aedes aegypti populations collected and their GPS coordinates.
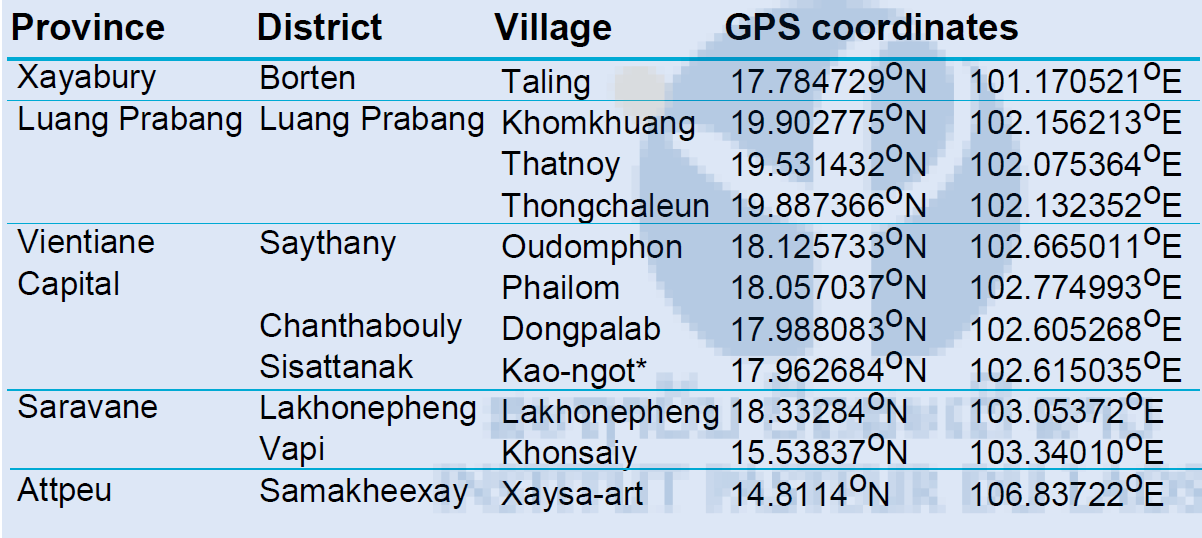
*IPL strain, collected at the Institut Pasteur du Laos

Figure 3. Larval collection of dengue vectors in Luang Prabang province, July 2015.
Morphological mosquito identification
For all the mosquito populations collected, larvae were reared until adults (F1 generation; Figure 4). After adult identification, mosquitoes obtained were separated by species and location. Only Aedes aegypti and Ae. albopictus were kept for breeding. Females mosquitoes were then blood fed using quail and the eggs obtained were kept for the larval and adult bioassays.

Figure 4. Colony room at the Institut Pasteur du Laos.
Insecticide resistance status
We tested the susceptibility of Aedes aegypti mosquitoes to a range of insecticides representative of those historically and currently used for mosquito control in Lao PDR (ie DDT, temephos, malathion, deltamethrin and permethrin). Larval and adult bioassays were be performed following WHO guidelines (WHO 2005, 2006; Figure 5).
Larval bioassays on Ae. aegypti were performed using late third- and early fourth-instar larvae of the field strains. For each bioassay, larvae of each strain were transferred to cups containing 99 mL of distilled water and 1 mL of the insecticide tested at the desired concentration. Five cups per concentration (25 larvae per cup) and 5–8 concentrations in the activity range of each insecticide were diluted in ethanol. USDA (reference susceptible strain) and field strains were considered as having different susceptibility to a given pesticide when the ratio between their LC50/95 or LD50/95 (resistance ratio: RR50/95) had confidence limits excluding the value of 1. A mosquito strain is considered susceptible when its value of RR50 is less than 5, moderately resistant when RR50 is between 5 and 10, and highly resistant when RR50 is over 10. Larval bioassays on the Ae. albopictus populations were run with diagnostic doses (WHO recommended) of deltamethrin (0.00132 mg/L), permethrin (0.014 mg/L), temephos (0.02 mg/L) and DDT (0.04 mg/L). Mortality was recorded after 24h. Following WHO criteria, a population is considered resistant if the mortality after 24 h is under 90%, resistance is suspected with mortality between 90 and 98% and a population is susceptible with mortality over 98%.
Adult bioassays were run using filter papers treated with diagnostic doses of deltamethrin (0.05%), permethrin (0.25% ), DDT (4%), and malathion (0.8%). Mortality resulting from tarsal contact with treated filter papers was measured using WHO test kits on adult mosquitoes of the different populations. Four batches of 25 non-blood-fed females (2–5 days of age) were introduced into holding tubes and maintained for 60 minutes at 27 ± 2°C and a relative humidity of 80 ± 10%. Insects were then transferred into the exposure tubes and placed vertically for 60 minutes under subdued light. Mortality was recorded 24 hours after exposure.

Figure 5. Larval and Adult bioassay.
Results of the larval bioassays on the Ae. aegypti populations are shown in Tables 2. For each strain and each insecticide, the dose mortality relationships were fitted by regression (P>0.05). For each bioassay, when control mortality was greater than 5% but less than 20%, then the observed mortalities were corrected using Abbott’s formula. The resistant ratios (RR) were calculated using the susceptible reference strain (USDA).
The Aedes aegypti populations from Taling (Xayabury province), Lakhonepheng (Saravane province), and Oudomphon village showed the highest resistant ratios to temephos (RR50>3). The other populations had significant RR50 and RR95 but like the previously cited cannot be considered as fully resistant. All the populations showed a tolerance to the insecticide deltamethrin, with significant RR50 (>6.25) and RR95 (>2.3). The populations from Saravane and Vientiane Capital (Phailom village) showed the highest resistance levels with RR50 of 17.5 and RR95 of 10.3, and RR50 of 18.8 and RR95 of 5.9, respectively. All the population tested against permethrin exhibited low to high resistance to the insecticide with RR50 between 2 and 15.2 and RR95 between 3.6 and 22.8. Aedes mosquitoes from Vientiane were the most resistant to permethrin. (RR50>13 and RR95>22). All the population tested against DDT exhibited high resistance to the insecticide with RR50 between 7.6 and 171.7 and RR95 between 6.9 and 89.3. Aedes mosquitoes from Taling in Xayabury province were the most resistant to DDT (RR50 = 171.7 and RR95 = 89.3).
Table 2: Resistance status of Aedes aegypti populations (larvae) against temephos, deltamethrin, permethrin and DDT.
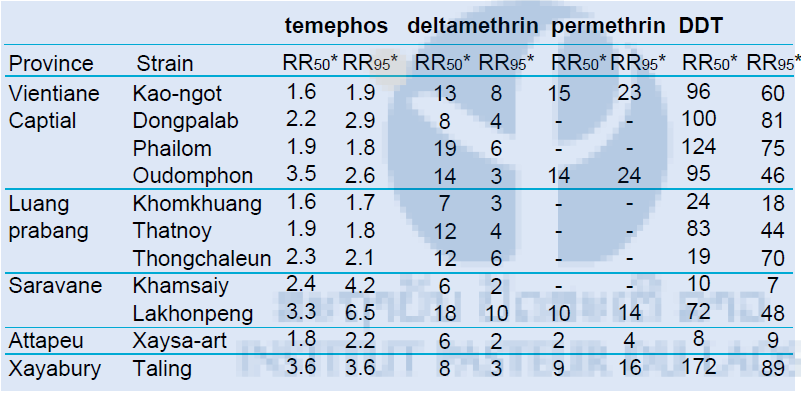
LC = Lethal concentration, *Resistant ratios = LC50 of wild strain / LC50 of USDA susceptible reference strain.
Results of the larval bioassays on the F1 Aedes albopictus populations are shown in Table 3. The result showed that all the populations tested were resistant to DDT, except in Oudomphon were resistance is suspected. All the populations were susceptible to permethrin. In Luang Prabang province, resistance to temephos is suspected. In Vientiane province, both population tested were resistant to temephos and the population from Suanmone was resistant to permethrin.
Table 3: Resistance status of Aedes albopictus (larvae) against DDT, temephos, malathion deltamethrin and permethrin
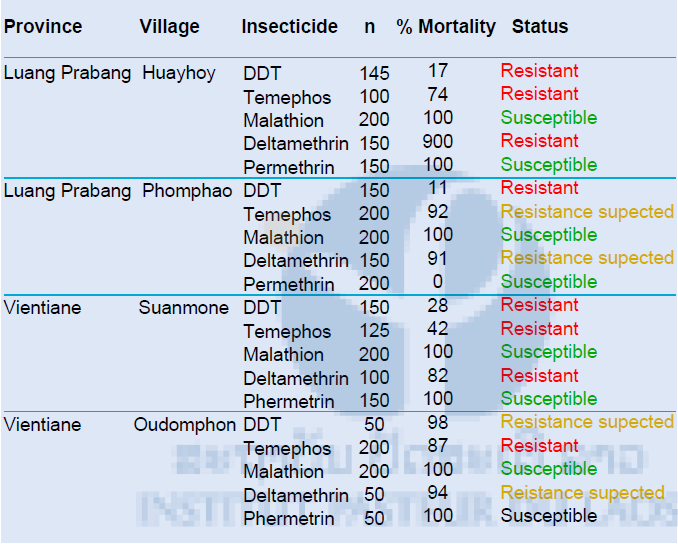
The diagnostic dosages of DDT, temephos, malathion, deltamethrin and permethrin used were 0.04 mg/L, 0.02 mg/L, 1 mg/L, 0.00132 mg/L and 0.014 mg/L, respectively.
The results of the adult bioassays are shown in Figure 6. Aedes aegypti populations from Vientiane, Luang Prabang, Saravane, Attapeu and Xayabury were tested. For each bioassay, when control mortality was greater than 5% but less than 20%, the observed mortality was corrected using Abbott’s formula. As expected, the susceptible reference strain (USDA) showed full susceptibility to the four insecticides tested. All the populations tested were highly resistant to the organochlorine DDT with resistance levels varying from zero to thirty-one percent mortality. The most resistant population was in Thongchaleun village in Luang Prabang province (0% mortality). All the populations tested against permethrin (pyrethroid family) presented high levels of resistance with mortality rates values between seven and eighty-three percent.
The most resistant population was in Thongchaleun village in Luang Prabang province (0% mortality). All the populations tested against permethrin (pyrethroid family) presented high levels of resistance with mortality rates values between seven and eighty-three percent. Most of the mosquito populations were susceptible to deltamethrin, which is also an insecticide from the pyrethroid family. Only one population, from Vientiane province (Dongpalab village), showed a moderate resistance to this insecticide (87% mortality). All the populations tested against malathion, an insecticide from the organophosphate family, were resistant to this insecticide (mortality<80%) or showed the beginnings of a resistance (mortality between 91% and 99%).
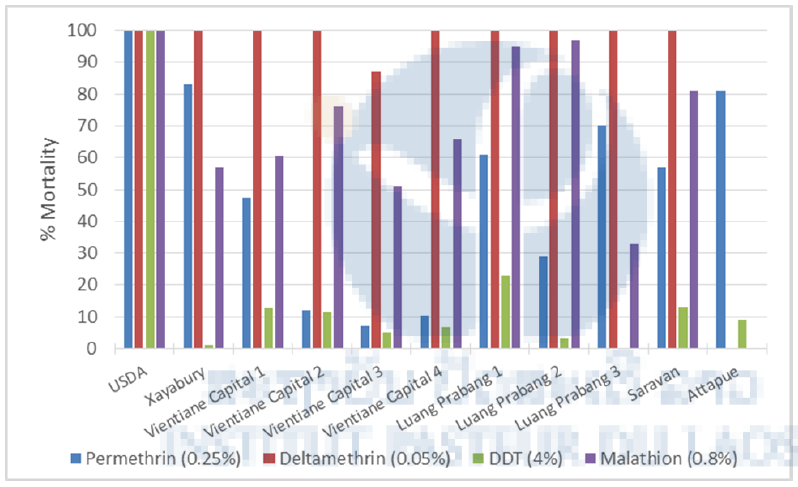
Figure 6. Mortality rates in WHO tube tests of Aedes aegypti from Lao PDR.
USDA: susceptible reference strain; Vientiane Capital 1: Kao-Gnot; V. Cap. 2: Phailom; V. Cap. 3: Dongpalab; V. Cap. 4: Oudomphon; Luang Prabang 1: Khomkhuang; LPB 2: Thatnoy; LPB 3: Thongchaleun.
Insecticide resistance mechanisms
Detoxification enzyme activities
The levels P450s, and the activities of CCEs and GSTs were assayed from single 3 day-old F1 females following the microplate methods described by Hemingway (WHO, 1998) and Brogdon (1997) on a spectrophotometer. Total protein quantification of mosquito homogenates were performed using Bradford reagent with bovine serum albumin as the standard protein in order to normalize enzyme activity levels by protein content. For P450s assays, the OD values were measured at 620 nm after 30 min incubation of individual mosquito homogenate. Nonspecific α- and β-CCEs activities were assayed by 10 min incubation of mosquito homogenate in each well with 100 μL of 3 mM napthyl acetate (either α- or β-) at room temperature and the OD values were measured at 540 nm. The activities were determined from α- or β-naphtol standard curves. Glutathione-S-transferases (GST) activity were measured in kinetic at 340 nm for 20 min, and the activity was expressed in nmoles GSH conjugated/min/mg protein.
All the populations tested showed significant higher quantity of P450s compared to the susceptible reference strain. Most of the populations except the population from Luang Prabang showed higher GSTs activities compared to the susceptible reference strain USDA. All the populations tested presented higher alpha and beta-Esterases activities than the susceptible strain (Figure 7).
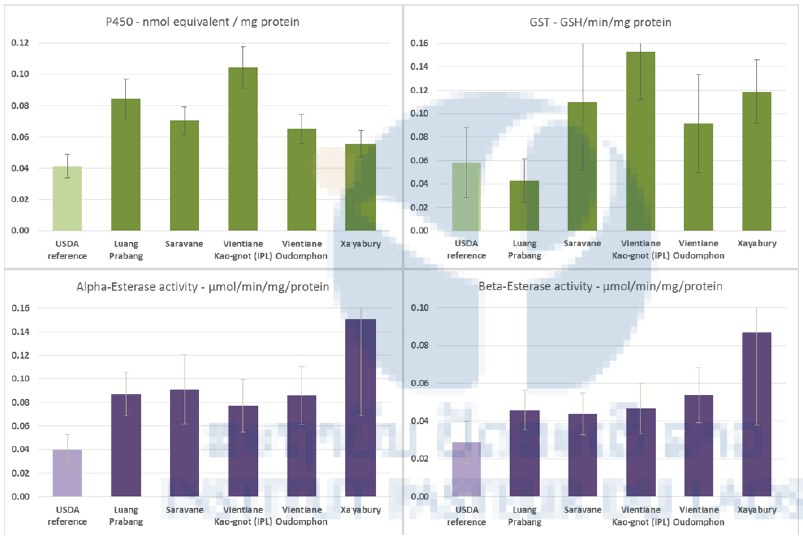
Figure 7. Global amount or activity of detoxification enzymes in Aedes aegypti larvae from field populations and the reference strain (USDA). Cytochrome P450 monooxygenases (P450s), Esterase (α and β-CCEs), and Glutathione-S transferases (GSTs). Sample sizes are 47 specimens/population). Confidence intervals are one standard deviation of the mean
KDR detection
Pyrethroid resistance can be due to metabolic detoxification and/or target site modification through non silent points of mutations (kdr) in the gene encoding for the Voltage-Gates Sodium Channel (Hemingway et al., 2004). In Aedes aegypti the known kdr mutations conferring pyrethroid resistance are located at loci 1016 (Val→Gly) and 1534 (Phe→Cys) (Brengue et al., 2003; Harris et al., 2010). Both mutations are present in Ae. aegypti in South-East Asia (Yanola et al., 2011; Kawada et al., 2009 and 2014) and are considered as relevant molecular markers to investigate pyrethroid resistance (Figure 8).
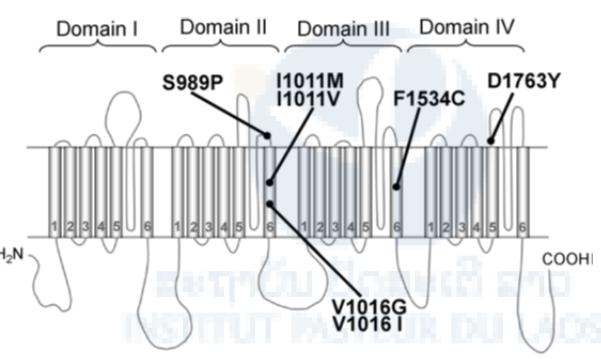
Figure 8. Diagram of the locations of possible kdr mutations found in Aedes aegypti. Point mutations in the voltage-gated sodium channel protein so far reported from pyrethroid-resistant Ae. aegypti are indicated.
Molecular detection of the V1016G substitution
The detection of V1016G mutation in the voltage-sensitive sodium channel has been performed by qPCR based on the High Resolution Melting (HRM) curve developed by Saaverda-Rodriguez et al. in 2007. A total of 1,076 females of Aedes aegypti coming from 11 field-caught populations were tested by real-time PCR to detect the presence of the V1016G and F1534C kdr mutations. The genotypic distribution and kdr frequency for the V1016G kdr mutation are shown in Table 4. The 1016G mutation was found at low frequency and range from 0 (Khonsaiy & Xaysa-art) to 0.36 (Dongpalab). Significant differences in the frequency of the 1016G allele were found between populations (pair-wise comparisons, P<0.05). All populations were at hardy Weinberg Equilibrium except Khomkhuang (P=0.0043) and Thongchaleun (P<0.001).
Table 4. Genotype distribution and allelic frequency of the V1016G kdr mutation
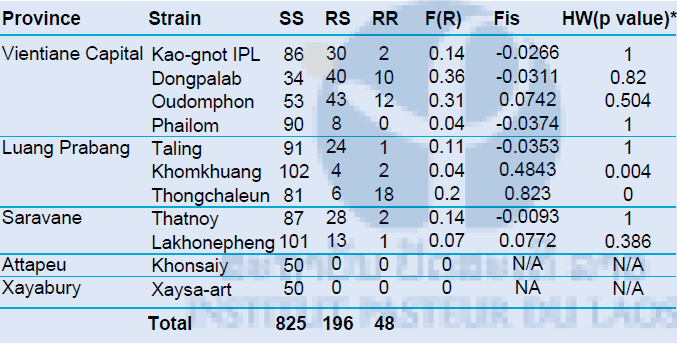
Hardy Weinberg equilibrium tested by exact-test Fisher. In bold, significant (p-value<0.05) population that showed difference from HW expectations.
Molecular detection of the F1534C substitution
The detection of the F1534C mutation on the voltage-sensitive sodium channel was based on the HRM method developed by Yanola and colleagues and adapted. by our team. The principle is the same as for the detection of the V1016G mutation The genotypic distribution and kdr frequency for the F1534C kdr mutation are shown in Table 10. The 1534C mutation was found at high frequency in all populations (>0.6) except at Xaysa-art where the prevalence was low and significantly different than all other populations (pair-wise comparison, P<0.05). Population genetic study showed that all populations were at hardy Weinberg equilibrium (HW, p-value>0.05).
Table 5. Genotype distribution and allelic frequency of the 1534C kdr mutation
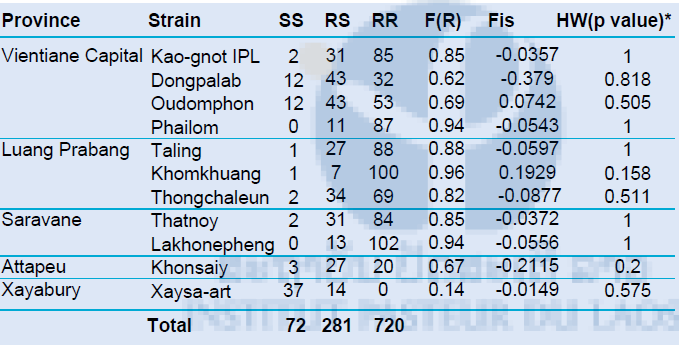
*Hardy Weinberg equilibrium tested by exact-test Fisher. In bold, significant (p-value<0.05) population that showed difference from HW expectations.
We also check for linkage disequilibrium between V1016G and F1534C in all populations (see Annex 1). Except for the two populations from Khonsaiy and Xaysa-art, there was non-random association of alleles at the two kdr loci (p-value<0.001). The non-independence interaction between kdr mutations may indicate selection process occurring at these two loci.
Genotype-phenotype association
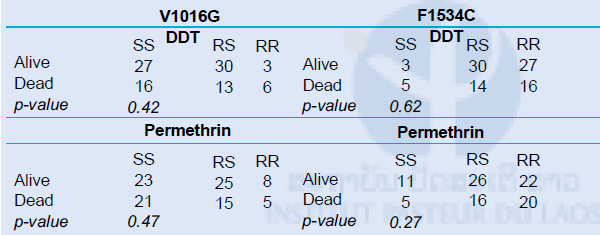
To assess the role of each mutation in permethrin and DDT resistance, we conducted a phenotype–genotype analysis by comparing the genotypic distribution of the V1016G and F1534C mutations between the dead and live mosquitoes after exposure to the two insticides (comparisons were made using Fisher’s exact test at 95% CI). The assumption was that a higher proportion of RR genotypes was expected in mosquitoes surviving the insecticide than the ones that died. Considering that the comparisons cannot be done with populations having fixed alleles (i.e. around 1 or 0), we selected populations having kdr allelic frequency for both mutations ranging from 0.3 to 0.7. Hence only two populations met this criteria, i.e., Oudomphon and Dongpalab.
The results are shown in Table 6. We did not find any significant difference between the frequency of kdr mutations (for both 1534C and 1016G) and the survival rate from DDT and permethrin (p>0.05). This may suggest that the kdr alleles may have no or only a minor role in the phenotype resistance observed to DDT and permethrin. However, the linkage disequilibrium between the two loci may explain this trend as the two loci are not independent from each other. Indeed, we noted negative association between the presence of homozygote-resistant genotypes for 1014G and the ones for the 1534C substitution. In other words, mosquitoes that exhibited RR genotypes for 1016G were mostly SS for the 1534C (and vice versa). This phenomenon may then bias the number of RR genotypes expected in each category (dead or alive). In addition, only two mosquitoes (of 1,076) were found homozygote resistant for both 1534C and 1016G, hence suggesting potential genetic cost associated with the double mutants.
Table 6. V106G and F1534C genotypic differentiation between dead and live mosquitoes for DDT and permethrin