SATREPS Project: Project for Development of Innovative Research Techniques in the Genetic Epidemiology of Malaria and Other Parasitic Diseases in the Lao PDR for Containment of Their Expanding Endemicity

Project coordinator: Dr. Shigeyuki KANO
Member of staff: Moritoshi IWAGAMI, Phonepadith KHATTIGNAVONG, Sengdeuane KEOMALAPHET, Pheovaly SOUNDALA, Lavy LORPHACHAN, Phonesavanh LUANGAMATH (March–June 2017) and other visiting scientists and students.
Background
Malaria, schistosomiasis (Schistosoma mekongi), and opisthorchiasis (Opisthorchis viverrini) are a tremendous health burden on the people of the Lao PDR. Although significant reductions in malaria transmission have been reported due to the large-scale distribution of insecticide-treated bed nets (ITNs) through the Global Fund to Fight AIDS, Tuberculosis and Malaria, strategies based on the scientific evidence have not been developed to deal with the genetic variations in parasite and vector populations, and drug-resistant malaria. Recently, artemisinin-resistant malaria was reported in southern provinces (Ashley et al., 2014; Ménard et al., 2016). Therefore, it is necessary to survey other provinces, especially the five southern provinces of the country, to monitor and contain the spread of drug-resistant malaria. The Lao Ministry of Health and the WHO have set a goal of eliminating malaria by 2030. To achieve this goal, we have to understand the real malaria situation, including drug-resistant malaria, prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency, and the prevalence of asymptomatic Plasmodium carriers, and develop elimination strategies.
Significant progress has been made in the past decades in the reduction of the prevalence of schistosomiasis (S. mekongi) in the endemic areas (approximately 200 villages) in Khong district and Mounlapamok district, Champasak province, Lao PDR, through preventive chemotherapy using praziquantel as the drug of choice once a year as well as health education in the community. However, current monitoring methods rely on the Kato–Katz stool examination whose sensitivity may not be sufficient to detect light S. mekongi infections. In 2017, WHO adopted a new strategy that accelerates the elimination of Asian schistosomiasis in the Western Pacific Region, i.e., transmission interruption by 2025 and eradication by 2030. One criteria of transmission interruption is “no new case of animal infection.” To achieve this goal, we need sensitive detection methods, such as DNA diagnostic methods (PCR and LAMP) and serological methods (ELISA), to monitor the prevalence of the disease precisely in the endemic areas.
Since opisthorchiasis (O. viverrini) is localized to the Lao PDR and surrounding countries, it is recognized as a neglected tropical diseases. Nevertheless, the prevalence of opisthorchiasis is estimated to be as high as 15–54% in the Lao PDR. Little information on the molecular/genetic epidemiology of opisthorchiasis is available to develop effective measures for prevention and diagnosis of the disease.
The Government of the Lao PDR requested the Japan International Cooperation Agency (JICA) to establish the Lao–Japan Joint Laboratory within Institut Pasteur du Laos (IPL) to conduct highly technological research on human malaria parasites (Plasmodium falciparum, P. vivax, and the monkey malaria parasite P. knowlesi) and human trematodiases (S. mekongi and O. viverrini). The joint research will concentrate on genetic epidemiological studies to detect and control the emergence and dissemination of these parasitic diseases. The project also contributes to the capacity development of researchers and technicians in the Lao PDR through training in field and lab work, seminars, and career development.
In order to carry out this project, the IPL in collaborating with the National Center for Global Health and Medicine (NCGM), Tokyo, Japan, the Center of Malariology, Parasitology and Entomology (CMPE), the National Institute of Public Health (NIOPH), and other Departments of the Ministry of Health, Lao PDR.
Objectives
The objectives of this project are (1) to develop more convenient and accurate methods (PCR methods, LAMP methods, etc.) for diagnosis of the diseases, (2) to monitor the temporal and spatial epidemiological situations of pathogens and vectors of the diseases, (3) to analyze mechanisms of emergence and expansion of drug-resistant malaria, especially artemisinin resistance, and (4) to analyze the G6PD activity of the Lao population for evaluation of the possible usage of primaquine (Howes et al., 2013), utilizing molecular biological techniques. Based on the scientific evidence obtained by this project, health education for the people will be strengthened and the endemicity of the diseases will be monitored together with the local Lao Ministry of Health officials. Research results will also be utilized in government services for the sustainable development of the Lao PDR.
Study period of the project
Five years (May 2014 to April 2019)
Project study sites
Malaria:
Savannakhet province, Salavan province, Sekong province, Attapeu province, Champasak province, Khammouane province, Phongsali province, Luang Prabang province.
Schistosomiasis (S. mekongi):
Khong district and Mounlapamok district, Champasak province.
Opisthorchiasis (O. viverrini):
Khammouane province, Champasak province.
Ethical clearance
The SATREPS project was approved by the National Ethic Committee for Health Research in the National Institute of Public Health (NIOPH), Ministry of Health, Lao PDR in 2014, 2015, 2016, and 2017 (extended each year).
Activities and Results from November 2016 to October 2017
We conducted nine field surveys on parasitic diseases, and three refresher training courses on malaria LAMP diagnostic methods for medical lab technicians in Luang Prabang, Savannakhet, and Champasak. We also conducted training on parasitic diseases for staff members in the Institute of Disease Prevention, Ministry of Defense and a staff member in the NIOPH. One of our Junior Scientists attended a training course on molecular parasitology at the National Center for Global Health and Medicine, Tokyo, Japan, in February 2017. This activity report summarizes our activities and the results of the SATREPS project in the Lao–Japan Parasitology lab from November 2016 to October 2017.
All field surveys were conducted in collaboration with the CMPE, Provincial Health Offices, and District Health Offices, Ministry of Health, Lao PDR.
Field surveys on malaria and schistosomiasis
1. Malaria and G6PD activity survey, Thapangthong, Phin, and Nong districts, Savannakhet province, 13th to 25th November 2016.
2. Schistosomiasis pre-survey with IPL Scientific Advisory Board Members, Khong district, Champasak province, 30th November to 1stDecember 2016.
3. Malaria and G6PD activity survey, Boun Nuea and Khoua districts, Phongsali province, 10th to 27th February 2017.
4. Malaria survey with Monash University, Melbourne, Australia, Pathoumphone district, Champasak province, 14th to 22nd March 2017.
5. Schistosomiasis snail survey, Khong district, Champasak province, 23rd to 30th April 2017.
6. Schistosomiasis spot-check survey, Don Khao village (students and adults) and Muang village (students), Khong district, Champasak province, 7th to 20th May 2017.
7. Malaria survey for blood sample collection in five southern provinces (Savannakhet, Salavan, Sekong, Champasak, and Attapeu), 21st May to 4th June 2017.
8. Schistosomiasis spot-check survey, Muang village (adults), Khong district, Champasak province, 20th to 27th August 2017.
9. Malaria and G6PD activity survey, Thapangthong district, Savannakhet province, 8th to 17th October 2016.
Field surveys
Written informed consent was obtained from all the participants prior to interviews and biological sample collection. The guardians of child participants consented to their participation. Three malaria diagnostic methods— microscopy, a rapid diagnostic test (RDT: Malaria Ag Pf / Pv, Standard Diagnostics, Inc. Republic of Korea), and a DNA diagnostic method—were used for detecting malaria parasites, malaria parasites antigen, and malaria parasites DNA, respectively. The Kato–Katz thick smear method of stool examination was used for detecting S. mekongi eggs in the stool samples. A DNA diagnostic method (LAMP method) was used for detecting S. mekongi DNA in the stool samples and snail samples. ELISA was also used for detecting S. mekongi antibodies in the blood samples.
1. Malaria and G6PD* activity survey in Savannakhet province in November 2016 and October 2017, and Phongsali province in February 2017.
*G6PD: Glucose-6-phosphate dehydrogenase
Target villages were randomly selected from malaria high-endemic villages (API >10) by District Health Offices (API: number of malaria cases per 1,000 population per year). Convenience sampling was performed with the support of the chief of village in each village.
First, socio-demographic data and clinical data were collected using a questionnaire form. Second, blood samples were collected by finger prick using a lancet. Malaria infection was checked by malaria rapid diagnostic test (RDT; Malaria Ag P.f /P.v, Standard Diagnostics, Inc., Republic of Korea) on site. When the villagers were diagnosed with malaria, a staff member from a district hospital or health center provided them with one pack of an antimalarial drug (Coartem) free of charge (supported by the Global Fund). Blood smears and dried blood samples on filter papers (FTATM Classic Card, GE Healthcare Life Sciences, WhatmanTM, UK) were also obtained from the villagers. The blood smears and dried blood samples are being analyzed by microscope and PCR, respectively, at IPL.
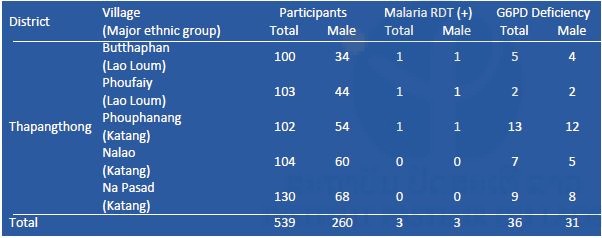
G6PD enzyme activity was measured on site by a G6PD assay kit (Dojindo Molecular Technologies, Inc., Japan) and a spectrophotometer (NanoDrop 2000c, Thermo Fisher Scientific, Inc., USA). Reagents for G6PD assay were stored in a portable liquid nitrogen tank (Vapor Shipper SC 4/3v, MVE Co., USA). When G6PD enzyme activity was less than 60% compared to a positive control (G6PD enzyme activity normal blood), the participants were considered to be G6PD deficient (WHO 1989). Mutation(s) of the G6PD gene among the G6PD-deficient participants will be examined by PCR and DNA sequencing by a Genetic Analyzer 3500XL (Applied Biosystems brand, Thermo Fisher Scientific, Inc., USA) at IPL. Results of the malaria RDT and G6PD assays are shown in Tables 1–3, respectively. Prevalence of G6PD deficiency in Savannakhet and Phongsali was 6.5% (60/927) and 2.6% (11/426), respectively. These results suggested that ethnic groups in the northern province of the country have fewer G6PD deficient alleles in their genome. In 2016, G6PD enzyme activity among villagers in Thapangthong district, Savannakhet province could not be measured (Table 1) because of a technical problem: the liquid nitrogen tank was broken on the way to the village.

Mr. Ken ONG, PhD student of the University of Tokyo as well as member of the SATREPS project, conducted a systematic review of literature about G6PD deficiency in the Greater Mekong Subregion and published a review article (Ong et al., 2017).

Table 1. Results of malaria RDT and G6PD assay in Savannakhet province in November 2016.
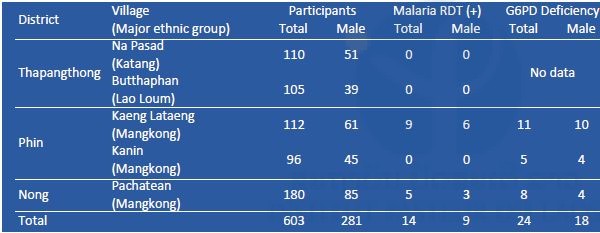
Table 2. Results of malaria RDT and G6PD assay in Phongsali province in February 2017.
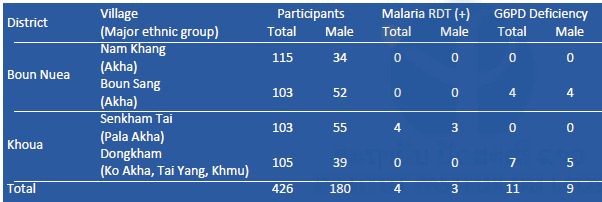
Table 3. Results of malaria RDT and G6PD assay in Savannakhet province in October 2017.
2. Malaria survey, Phatounphone district, Champasak province, 14thto 22nd March 2017.
Target villages were randomly selected from malaria high-endemic villages (API >10) by District Health Offices (API: number of malaria cases per 1,000 population per year). Convenience sampling was performed with the support of the chief of village in each village.
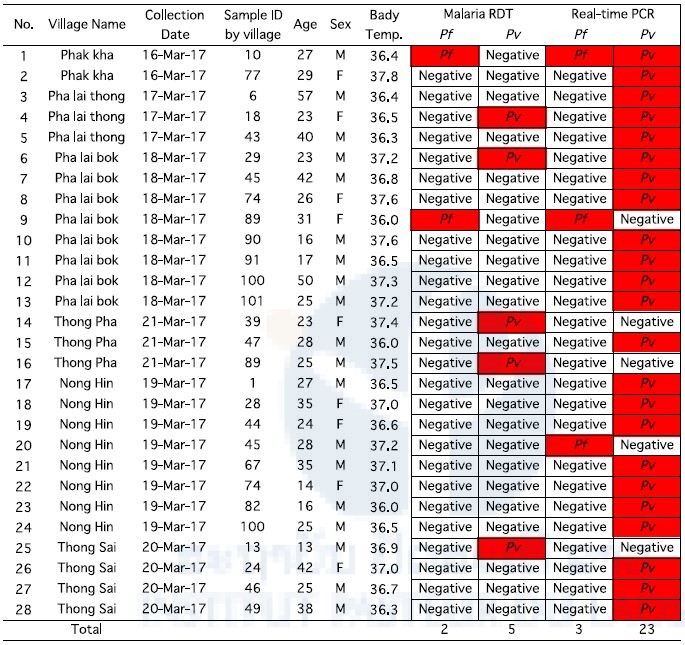
Five hundred ninety-five villagers participated in this survey. Blood samples were collected by finger prick using a lancet. Malaria infection was checked by malaria RDT on site. When the villagers were diagnosed with malaria, a staff member from a district hospital or health center provided them with one pack of an antimalarial drug (Coartem) free of charge. Blood smears and dried blood samples on filter papers were also obtained from the villagers. They were analyzed by microscope and PCR, respectively, at IPL. A summary of the malaria survey by malaria RDT and PCR are shown in Table 4. Overall, 208 were males and 387 were females. A total of 28 participants were malaria positive: 19 males and 9 females.
Table 4. Results of malaria RDT and PCR in Champasak province in March 2017.
3. Schistosomiasis snail survey, Khong district, Champasak province, 23th to 30th April 2017
We collected approximately 5,500 intermediate snail hosts, Neotricula aperta, at six riversides in Khon village on Khon Island in April 2017 (Figure 1).
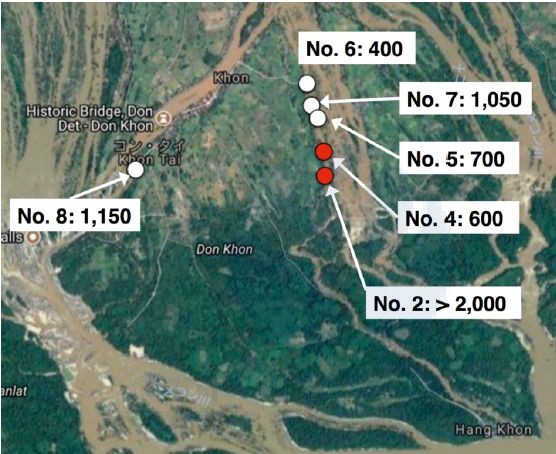
Figure 1. Snail sampling locations in Don Khon, Khong district, Champasak province in April 2017. Numbers show numbers of snails collected. White circles show locations where only S. mekongi negative snails were collected, whereas red circles show locations where samples collected included some S. mekongi positive snails. (Google Maps Co.)

All the snail samples were preserved in 70% ethanol and sent to Dr. Kumagai’s laboratory (Dr. Kumagai is a member of the SATREPS project), Tokyo Medical and Dental University (TMDU), Tokyo, Japan on 19th June 2017. After obtaining an authorization for shipping from the Misnistry of Health. Dr. Kumagai developed the S. mekongi LAMP diagnostic method.
Infection status of the snails with S. mekongi was evaluated by the LAMP method using pooled DNA samples. DNA was extracted from the pooled snail samples with 50 snails per pool by the alkali boiling method with the exception of location No. 2 where 1,000 snails were pooled with 50 snails per pool (20 pools) and another 1,000 snails were pooled with 200 snails per pool (5 pools). By the LAMP method S. mekongi DNA was detected from some of the pooled snail samples collected from locations No. 2 and No. 4. The estimated prevalence of S. mekongi in the snails was 0.10 in location No. 2 and 0.17 in location No. 4 (in preparation for publication). In the previous study, we detected S. mekongi-positive snails from these two locations in April 2016. Moreover, we also detected S. mekongi patients by the LAMP method using stool samples near these locations in April 2016.
4. Schistosomiasis spot-check survey, Khong district, Champasak province, in May and August 2017.
Participants in this survey were randomly selected using a household list for the adult population (15–65 years old) and a student list in a school for students (6–14 years old). For each village, 100 adults and 100 students were included for the survey. If the number of students in a school was less than 100, all students at the school were included in the survey.
We conducted schistosomiasis mekongi surveys in two spot-check villages: Muang and Don Khao, Khong district, Champasak province, in May and August 2017. These surveys are a part of the Lao national survey on schistosomiasis to achieve transmission interruption by 2025 and elimination by 2030. To monitor the prevalence of schistosomiasis, we performed stool examination by the Kato–Katz thick smear method with 2 samples/person and 2 smears/sample, i.e. total 4 smears/person. Although WHO recommends the Kato–Katz method as a standard method for detecting parasite eggs in stool samples, the sensitivity of the Kato–Katz method was not enough to detect light levels of infection. Therefore, we also performed ELISA to detect S. mekongi antibodies in blood samples. A total of 258 blood samples (approximately 100 µL) were collected from villagers in the two spot-check villages by finger-prick on filter papers (Table 5).
By the Kato–Katz method, all smears were negative for S. mekongi eggs. The ELISA will be performed at IPL using S. japonicum soluble egg antigen.
Table 5. Summary of schistosomiasis spot-check survey, Khong district, Champasak province in May and August 2017.
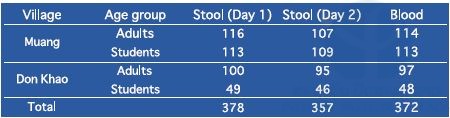
All the stool samples were negative for S. mekongi eggs by the Kato–Katz method. Students: 6–14 years old, Adults: 15–65 years old.
5. Blood sample collection from malaria suspected patients in the five southern provinces (Savannakhet, Salavan, Sekong, Attapeu, and Champasak), May to June 2017.
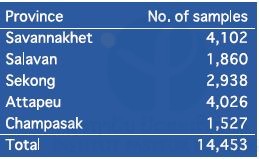
We collected 14,453 dried blood samples on filter papers (FTATM Classic Card, GE Healthcare Life Sciences, WhatmanTM, UK) from malaria suspected patients who visited 156 public health facilities in the five southern provinces from October 2016 to May or June 2017. The number of blood samples in each province is shown in Table 6. Malaria DNA analysis will be conducted at IPL using those filter paper samples. First, malaria parasite species will be identified by nested real-time PCR. Second, mutations of drug-resistant genes, such as the k13 propeller gene, or pfcrt gene, will be examined using P. falciparum-positive samples.
Table 6. Summary of blood samples collected from malaria suspected patients in the five southern provinces from October 2016 to May or June 2017.
6. Drug resistance in P. falciparum in the Lao PDR
We examined mutation(s) of the k13 propeller gene of P. falciparum that is associated with artemisinin resistance using isolates collected from the five southern provinces from October 2015 to April 2016. In this sampling period, we collected 2,409 blood samples on filter papers from malaria patients who visited 156 public health facilities in the five southern provinces. First, malaria species were identified by real-time PCR, and 1,151 samples were proved to be P. falciparum mono-infection or mixed infection with P. falciparum and P. vivax. Mutation(s) of the k13 of the 1,151 samples were examined by PCR and DNA sequencing. Overall, 55.5% (639/1,151) of the samples possessed non-synonymous mutations in the k13 gene. Frequencies of the k13 mutations in the five provinces were as follows: Savannakhet 28.0% (71/254), Salavan 58.3%(126/216), Sekong 38.6% (54/140), Attapeu 69.1% (85/123), and Champasak 72.5% (303/418) (Figure 2). These distribution patterns of the k13 mutations suggest that the resistant isolates may have been introduced by human movement from Thailand/Cambodia and subsequently migrated northward in the Lao PDR (in preparation for publication). Artemisinin resistance was observed to be rapidly increasing and spreading northwards into the Lao PDR. The Lao government and the international community should make dedicated efforts to contain the spread of k13-mutated P. falciparum in the Lao PDR by targeting high-risk populations.
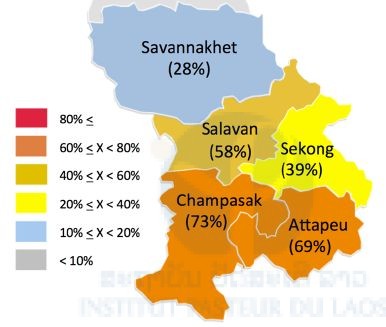
Figure 2. Percentages of the k13 mutations in the five southern provinces in the Lao PDR.
7. Risk factors of malaria infection in the Lao PDR
We reanalyzed data from a malaria field survey in Attapeu province in May 2015. In this study, we collected blood samples from 719 villagers who lived in 6 malaria-endemic villages in 3 districts (Xaysetha, Sanamxay, and Phouvong) in Attapeu province. Social-demographic and clinical data were also obtained using a questionnaire forms. Malaria infection status was determined by PCR which revealed parasitemia in 6.5% of the villagers (47/719), and only one showed a fever (≥37.5oC) at the time of survey. The dominant species was P. vivax (87.2%, 41/47). Multivariate logistic regression analyses that adjusted for the effects of variables were conducted to estimate the association between variables and Plasmodium infection and asymptomatic Plasmodium infection using SPSS version 18.0 (SPSS INC., Chicago, IL, USA). All variables with a P-value of 0.20 from univariate analysis were entered into a multivariate logistic regression analysis. Multicollinearity among all independent variables was tested before logistic regression.
Multivariate analyses showed that being male, a soldier, and Lao Loum were risk factors for Plasmodium infections (Table 7).
Table 7. Multivariate logistic regression analyses to identify risk factors for Plasmodium infections in Attapeu province.
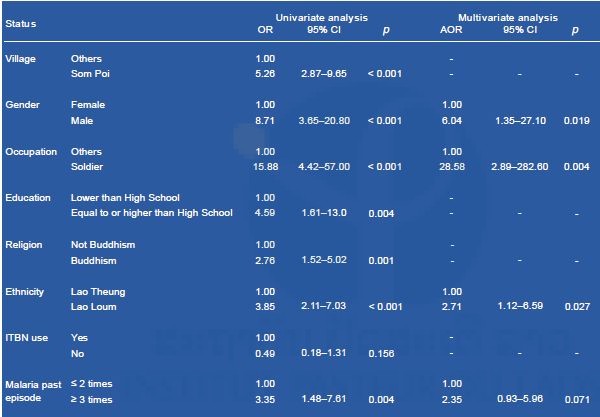
OR, odds ratio; CI, confidence interval; AOR, adjusted odds ratio; ITBN, insecticide-treated bed net.
Multivariate analyses showed that having a history of multiple malaria episodes (≥3 times) was a risk factor for asymptomatic (cryptic) Plasmodium infections (Table 8). To achieve malaria elimination in the Lao PDR, highly sensitive diagnostic tests, including PCR-based diagnostic methods, should be used, and plans targeting high-risk populations and the elimination of P. vivax should be designed and implemented (accepted for publication in PLOS NTD).
Ms. Nouhak INTHAVONG, master course student of the University of the Ryukyus as well as a member of the SATREPS project also conducted a malaria field survey in Xepon district, Savannakhet province, from December 2016 to January 2017, to identify risk factors associated with Plasmodium infection. Her study showed that night-time working and sleeping behaviors in the forest and the presence of an opening in the house exterior walls (size larger than A4 sheet) were significantly associated with Plasmodium infection in the study villages (Inthavong et al., 2017). The Lao National Malaria Control Program should recommend that villagers use personal protection when working and sleeping in the forest and to reduce the number of such openings in house exterior walls.
Table 8. Multivariate logistic regression analyses to identify risk factors for cryptic Plasmodium infections in Attapeu province.
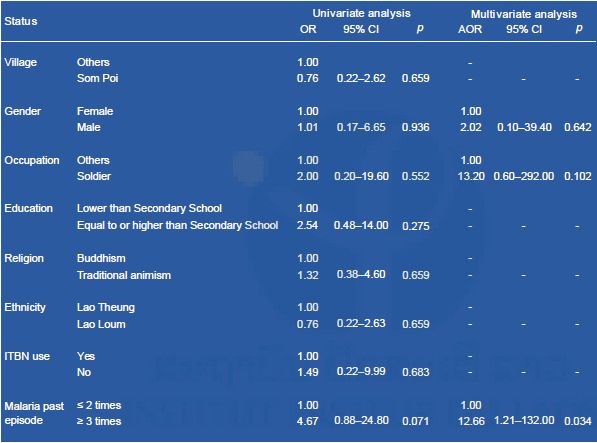
8. Prevalence of Schistosoma mekongi by ELISA in seven sentinel sites and two spot-check sites in Khong district and Mounlapamok district, Champasak province, in 2016.
In 2016, IPL joined the Lao national survey on schistosomiasis in seven sentinel sites and two spot-check sites in Khong district and Mounlapamok district, Champasak province. In this survey, we collected and examined stool samples (n=1,131) and blood samples (n=1,754) from villagers: adults (15–65 years old) and students (6–14 years old) in the nine target villages, to monitor the prevalence of schistosomiasis in the endemic areas. Villagers (participants) were randomly selected. The prevalence of schistosomiasis among villagers by the Kato–Katz stool examination is shown in Table 9. The prevalence of schistosomiasis in the nine target villages ranged from 0.0% to 8.3% without heavy infection using the Kato–Katz method. In this survey, we attempted to collect two stool samples (day 1 and day 2) per villager, to increase the sensitivity of the Kato–Katz stool examination. When two stool samples were not obtained from the participant, we excluded the data of the participant from the final result. On the other hand, the prevalence of schistosomiasis among villagers by ELISA is shown in Table 10. ELISA results (2.8% to 53.3%) were much higher than those of the Kato–Katz method.
Generally, the Schistosoma antibody remains in a human body for certain period (up to 10 years) after treatment by praziquantel. Therefore, some of the ELISA positive cases represent a history of previous infection by S. mekongi. The Lao Ministry of Health has conducted mass drug administration (preventive chemotherapy) with praziquantel in 202 villages in the Khong district and Mounlapamok district, Champasak province, once a year with health education. If new infection or re-infection does not occur among the villagers, both the antibody titer of the villagers (previous patients) and the antibody positivity rate in the villages would decrease gradually.
According to a report from Vonghachack et al. 2017, 14.7% of dogs (10/68) were infected with S. mekongi in Khong district, Champasak province (Vonghachack et al., 2017). This study was conducted by convenience sampling between October 2011 and August 2012. Therefore, the prevalence of S. mekongi among dogs and some other animals that would have contact with water (e.g. water buffalos, cattle, and pigs) should be evaluated by a random sampling with a statistically sound sample size.
Table 9. Prevalence of schistosomiasis mekongi among villagers in Khong and Mounlapamok districts, Champasak province in 2016 by Kato–Katz stool examination.
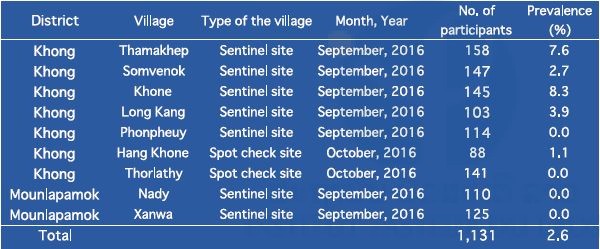
Table 10. Prevalence of schistosomiasis mekongi among villagers in Khong and Mounlapamok districts, Champasak province in 2016 by ELISA.
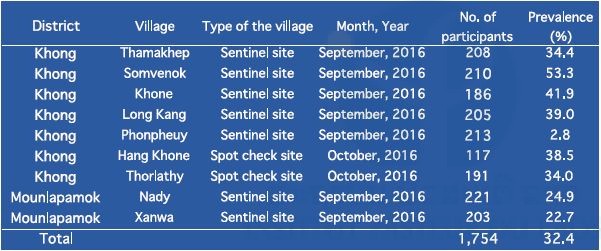
Schistosoma antibody-positive implied both current infections and a history of previous infections by Schistosoma.
9. Detection of Opisthorchis viverrini from freshwater fish collected from opisthorchiasis endemic area in Yommalath district, Khammouane province by LAMP method in 2017.
Identification of Opisthorchis viverrini by classical methods, such as microscopic examination for detecting O. viverrini eggs in stool samples or O. viverrini metacercariae in freshwater fish is very difficult because there are several other trematoda parasites that morphologically resemble O. viverrini (Yoon et al., 2014; Sohn et al., 2014; Sayasone et al., 2015; Onsurathum et al., 2016). In this study, we attempted to identify O. viverrini metacercariae by the LAMP method using metacercariae obtained from freshwater fish. Freshwater fish were collected from an opisthorchiasis endemic area in Yommalath district, Khammouane province in April and June 2017.
First, freshwater fish (scaled fish: 10–13cm in length) were cut into small pieces using a food processor and digested with artificial gastric acid juice (0.5% pepsin, 0.7% HCl, pH 1.5). A mixture of the fish and the gastric acid juice was incubated for 1–2 hours at 37oC. Second, a supernatant of the digested mixture was discarded and the sediment was filtrated with a strainer to remove bones and scales. Third, the sediment was washed with water and filtrated by nylon mesh (φ57μm). The captured paste was washed with water until the supernatant became clear. Metacercariae were observed under a light microscope and collected one by one into a plastic tube for DNA analysis.
DNA was extracted by the alkali-boiling method. The extracted DNA was used for the LAMP assay. For the LAMP assay, we used O. viverrini specific primer sets as well as Haplorchis taichui specific primer sets. H. taichui is one of the trematoda parasites with a morphological resemblance to O. viverrini and often coexists in the same endemic areas. Results of the LAMP assay are shown in Table 11.
Most of the metacercariae obtained from the fish proved to be H. taichui or neither O. viverrini nor H. taichui. Many metacercariae were found from freshwater fish but some of the species were unknown. In the previous LAMP assay for O. viverrini using 119 stool samples from Mounlapamok district in 2015, the O. viverrini positivity rate as measured by LAMP (47.6%) was lower than that by the Kato–Katz method (76.5%). These results suggest that the Kato–Katz method may overestimate O. viverrini infection rates in the stool samples.
Table 11. Result of the LAMP assay of metacercariae obtained from freshwater fish.
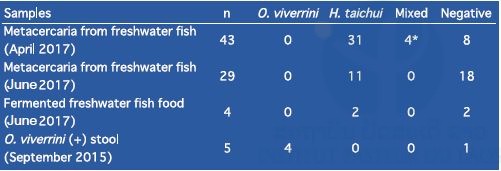
*Due to lack of skills, the possibility exists that there was more than one metacercaria per tube for DNA extraction.
Refresher training on malaria LAMP diagnostic method in the three provinces
Refresher training courses on the malaria LAMP diagnostic method (the Eiken Chemical Co., Japan) for medical lab technicians in malaria sections, Provincial Health Departments in the three provinces (Luang Prabang, Savannakhet and Champasak) where the LAMP system was implemented in 2016 were conducted with an expert of the Eiken Chemical Co. in 2017. Eight medical lab technicians (three in Luang Prabang, two in Savannakhet and three in Champasak) participated in the refresher training courses.
In the training, we reviewed all instructions including DNA extraction by LoopampTM PURE DNA Extraction Kit and handling the malaria LAMP kit: the LoopampTM MALARIA Pan/Pf Detection Kit (the Eiken Chemical Co., Japan). We also demonstrated how to clean the lab and the lab equipment with 5% sodium hypochlorite to prevent DNA contamination. In Luang Prabang, from September 2016 to January 2017, nine assays were performed for malaria suspected patients and all cases were negative for malaria DNA (the LAMP system was installed in September 2016). In Savannakhet, from August 2016 to March 2017, 31 assays were performed and all cases were negative for malaria DNA (the system was installed in August 2016). In Champasak, from August 2016 to March 2017, no assay was performed (the system was installed in August 2016). The Champasak malaria section is located in the Champasak Provincial Hospital. Thus, they work very closely with the hospital. The staff members in the Champasak malaria section told us that they did not perform the LAMP assay because the hospital did not order the malaria LAMP assay. We need to discuss this issue with Champasak Provincial Hospital to utilize the malaria LAMP assay for malaria diagnosis.

Other training
We conducted training on parasitic diseases for two lab staff in the Institute of Disease Prevention, Ministry of Defense, twice weekly, from January to March 2017. Both lectures and practices were conducted. In April 2017, we also conducted one week of laboratory training on parasitic diseases (mainly malaria DNA work) for a staff member in the NIOPH. This NIOPH staff member is currently a PhD student in the Swiss Tropical and Public Health Institute. Dr. Sengdeuane KEOMALAPHET, a Junior Scientist at IPL, attended a training course on molecular parasitology at the Department of Tropical Medicine and Malaria, Research Institute, NCGM, Tokyo, Japan, from 1st February to 2nd March 2017.
Summary
We conducted nine field surveys on parasitic diseases and three refresher training courses on the malaria LAMP diagnostic method for medical lab technicians in the three provinces (Luang Prabang, Savannakhet, and Champasak). This activity report summarizes our activities and the results of the SATREPS project in the Lao–Japan Parasitology Lab from November 2016 to October 2017.
Malaria study
We conducted G6PD surveys in Savannakhet province in November 2016 and October 2017 and Phongsali province in February 2017. The prevalence of G6PD deficiency in Savannakhet and Phongsali was 6.5% (60/927) and 2.6% (11/426), respectively. These results suggested that ethnic groups in the northern province of the country have fewer G6PD deficient alleles in their genome.
We examined 1,151 P. falciparum isolates collected from the five southern provinces in the country from October 2015 to April 2016. Overall, 55.5% (639/1,151) of the isolates possessed mutations (mainly C580Y) in the k13 gene that are associated with artemisinin resistance. The frequency was the highest in the southern-most provinces of the country (Champasak and Attapeu) and decreased toward the north (Savannakhet). These distribution patterns of the k13 mutations suggest that the resistant isolates may have been introduced by human movement from Thailand/Cambodia and subsequently migrated northward in the Lao PDR. Careful monitoring of the efficacy of artemisinin is necessary, especially in the southern-most provinces.
We estimated risk factors associated with Plasmodium infection by multivariate logistic regression analyses using data from malaria field surveys in Attapeu province in May 2015 and Savannakhet province during December 2016 to to January 2017. In the Attapeu survey, risk factors associated with Plasmodium infection were being male, a soldier, and Lao Loum. In the Savannakhet survey, night-time working and sleeping behavior in the forest, and the presence of openings in the exterior walls of house were significantly associated with Plasmodium infection in the study villages. To achieve malaria elimination by 2030, the Lao government should design and implement effective plans targeting malaria high-risk populations, such as males, soldiers, and forest workers.
Schistosomiasis study
We developed the Schistosoma mekongi LAMP diagnostic method for detecting S. mekongi DNA. We examined approximately 5,500 freshwater snails (Neotricula aperta), an intermediate host of S. mekongi, collected from six riversides on Khon Island, Khong district, Champasak province in April 2017, by the LAMP method. This analysis estimated that the positivity rates of the snail samples with S. mekongi were 0.10% to 0.17% at two riversides and 0.00% at the other four. The two riversides where S. mekongi-infected snails were found were close to the houses of S. mekongi patients who were diagnosed by the LAMP method using stool samples in April 2016.
Results of the national survey on schistosomiasis in 2016 showed that the prevalence of schistosomiasis ranged from 0.0% to 8.3% without heavy infection in seven sentinel site villages and two spot-check villages using the Kato–Katz method for parasite egg detection in stool samples (n=1,131). On the other hand, the prevalence of S. mekongi antibody (2.8% to 53.3%) was much higher using the ELISA method for detecting Schistosoma antibodies in blood samples from the same participants (n=1,755).
Opisthorchiasis study
We attempted to detect O. viverrini metacercariae DNA from freshwater fish collected from Yommalath district, Khammouane province where opisthorchiasis was highly endemic in April and June 2017. We digested 76 freshwater fish (scaled fish: 10–13cm in length) with artificial gastric acid juice. Metacercariae were obtained by filtration and washing from the digested sediments. Species of metacercariae were identified by the LAMP method using a DNA template extracted from the single metacercaria with O. viverrini– and H. taichui-specific primer sets. Only 4 samples were positive for O. viverrini DNA and H. taichui DNA, whereas 44 samples were positive for H. taichui DNA. The remaining 28 samples were unknown species. This result suggests that several trematoda parasites coexist in freshwater fish in Yommalath district and the prevalence of O. viverrini in freshwater fish might be overestimated.
Training courses
In 2017, we conducted two-day refresher training courses on the malaria LAMP method for medical lab technicians in the malaria section in Provincial Health Departments in the three provinces (Luang Prabang, Savannakhet, and Champasak) in January and March 2017. Dr. Sengdeuane KEOMALAPHET, Junior Scientist of IPL, attended a training course on molecular parasitology at Research Institute, NCGM, Tokyo, Japan in February 2017.
Partners
+ Center of Malariology, Parasitology and Entomology (CMPE), Ministry of Health, Vientiane Capital, Lao PDR
+ National Institute of Public Health (NIOPH), Ministry of Health, Vientiane Capital, Lao PDR
+ Department of Communicable Diseases Control (DCDC), Ministry of Health, Vientiane Capital, Lao PDR
+ Department of Training and Research, Ministry of Health, (DTR) Vientiane Capital, Lao PDR
+ Department of Hygiene and Health Promotion (DHHP), Ministry of Health, Vientiane Capital, Lao PDR
+ National Center for Global Health and Medicine (NCGM), Tokyo, Japan
+ Department of Community and Global Health, School of International Health, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
+ Department of Global Health, Graduate School of Health Sciences, University of the Ryukyus, Okinawa, Japan
+ Department of Molecular and Cellular Parasitology, Juntendo University School of Medicine, Tokyo, Japan
+ Section of Environmental Parasitology, Department of International Health Development, Division of Public Health, Graduate School, Tokyo Medical and Dental University, Tokyo, Japan.
Scientific communications
Publications:
Inthavong N, Nonaka D, Kounnavong S, Iwagami M, Phommala S, Kobayashi J, Hongvanthong B, Pongvongsa T, Brey PT, Kano S. Individual and household factors associated with incidences of village malaria in Xepon district, Savannakhet province, Lao PDR. Tropical Medicine and Health, 45: 36, 2017.
Ong KIC, Kosugi H, Thoeun S, Araki H, Thandar MM, Iwagami M, Hongvanthong B, Brey PT, Kano S, Jimba M. Systematic review of the clinical manifestations of glucose-6-phosphate dehydrogenase deficiency in the Greater Mekong Subregion: implications for malaria elimination and beyond. BMJ Global Health, 2: e000415, 2017.
Ménard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, Rahim-Awab G, Barnadas C, Berry A, Boum Y, Bustos MD, Cao J, Chen JH, Collet L, Cui L, Thakur GD, Dieye A, Djallé D, Dorkenoo MA, Eboumbou-Moukoko CE, Espino FE, Fandeur T, Ferreira-da-Cruz MF, Fola AA, Fuehrer HP, Hassan AM, Herrera S, Hongvanthong B, Houzé S, Ibrahim ML, Jahirul-Karim M, Jiang L, Kano S, Ali-Khan W, Khanthavong M, Kremsner PG, Lacerda M, Leang R, Leelawong M, Li M, Lin K, Mazarati JB, Ménard S, Morlais I, Muhindo-Mavoko H, Musset L, Na-Bangchang K, Nambozi M, Niaré K, Noedl H, Ouédraogo JB, Pillai DR, Pradines B, Quang-Phuc B, Ramharter M, Randrianarivelojosia M, Sattabongkot J, Sheikh-Omar A, Silué KD, Sirima SB, Sutherland C, Syafruddin D, Tahar R, Tang LH, Touré OA, Tshibangu-wa-Tshibangu P, Vigan-Womas I, Warsame M, Wini L, Zakeri S, Kim S, Eam R, Berne L, Khean C, Chy S, Ken M, Loch K, Canier L, Duru V, Legrand E, Barale JC, Stokes B, Straimer J, Witkowski B, Fidock DA, Rogier C, Ringwald P, Ariey F, Mercereau-Puijalon O; KARMA Consortium. A Worldwide Map of Plasmodium falciparum Artemisinin Resistance. New England Journal of Medicine. 374: 2453–2464, 2016.
Pongvongsa T, Nonaka D, Iwagami M, Nakatsu M, Phongmany P, Nishimoto F, Kobayashi J, Hongvanthon B, Brey PT, Moji K, Mita T, Kano S. Household clustering of asymptomatic malaria infections in Xepon district, Savannakhet province, Lao PDR. Malaria Journal. 15: 508, 2016.
Iwagami M, Keomalaphet S, Khattignavong P, Soundala P, Lorphachan L, Matsumoto-Takahashi E, Strobel M, Reinharz D, Phommasansack M, Hongvanthong B, Brey PT, Kano S. The detection of cryptic Plasmodium infection among villagers in Attapeu province, Lao PDR. (accepted for publication in PLOS Neglected Tropical Diseases)
In review for publication
Iwagami M, Tangpukdee N, Wilairatana P, Krudsood S, Dao LD, Nakazawa S, Sinuon M, Socheat D, Yasuoka J, Jimba M, Watanabe H, Kobayashi J, Toma H, Vanisaveth V, Hongvanthong V, Brey PT, Kano S. Pfcrt genotypes and microsatellite DNA polymorphisms flanking the gene on Plasmodium falciparum suggest different chloroquine selective pressure among populations in the Greater Mekong Subregion.
Iwagami M, Nakatsu M, Khattignavong P, Soundala P, Lorphachan L, Keomalaphet S, Xangsayalath P, Kawai S, Hongvanthong B, Brey PT, Kano S. First case of human infection with Plasmodium knowlesi in Laos
Oral presentations:
Kano S, Keomalaphet S, Khattignavong P, Soundala P, Lorphachan L, Phommasansack M, Hongvanthong B, Brey PT, Iwagami M. The detection of hidden Plasmodium infection in Laos. The 4th International Conference on Tropical Medicine, Infectious Diseases and Public Health, Edinburgh, Scotland, September 7th–8th, 2017.
Phoutnalong V, Nonaka D, Hongvanthong B, Iwagami M, Kounnavong S, Kobayashi J, Brey PT, Kano S. Malaria epidemiology trend in Lao PDR between 2009 and 2016. The 11th National Health Research Forum, Vientiane Capital, Lao PDR, October 24th–25th, 2017.
Inthavong N, Nonaka D, Kounnavong S, Iwagami M, Phommala S, Kobayashi J, Hongvanthong B, Pongvongsa, Brey PT, Kano S. Individual and household factors associated with incidences of village malaria in Xepon district, Savannakhet province, Lao PDR. The 11th National Health Research Forum, Vientiane Capital, Lao PDR, October 24th–25th, 2017.
Keomalaphet S, Khattignavong P, Soundala P, Lorphachan L, Iwagami M, Matsumoto-Takahashi E, Hongvanthong B, Brey PT, Kano S. Detection of cryptic Plasmodium infection among villagers in Attapeu province, Lao PDR. The 11th National Health Research Forum, Vientiane Capital, Lao PDR, October 24th–25th, 2017.
Azuma M, Nonaka D, Iwagami M, Kobayashi J, Kounnavong S, Hongvanthong B, Pongvongsa T, Brey PT, Kano S. Active case detection of people with asymptomatic malaria using snowball sampling method in Thapangthong district, Savannakhet, Lao PDR: a pilot study. The 11th National Health Research Forum, Vientiane Capital, Lao PDR, October 24th–25th, 2017.
Iwagami M, Nakatsu M, Matsumoto-Takahashi E, Keomalaphet S, Khattignavong P, Soundala P, Lorphachan L, Hongvanthong B, Brey PT, Kano S. Analyses on k13 propeller gene mutations in Plasmodium falciparum revealed spread of artemisinin resistance in five Southern provinces of Lao PDR in 2015-2016. The 11th National Health Research Forum, Vientiane Capital, Lao PDR, October 24th–25th, 2017.
Kumagai T, Iwagami M, Yamabe M, Keomalaphet S, Khattignavong P, Lorphacan L, Soundala P, Hongvanthong B, Ohta N, Brey PT, Kano S. The evaluations of the LAMP method and Kato-Katz method for risk mapping of Schistosoma mekongi infections in Champasak province. The 17th Regional Network on Asian Schistosomiasis and Other Helminth Zoonosis, Vientiane Capital, Lao PDR, October 24th–25th, 2017.
Khattignavong P, Keomalaphet S, Lorphacan L, Soundala P, Kumagai T, Iwagami M, Hongvanthong B, Brey PT, Kano S. Schistosomiasis prevalence based on ELISA technique in Champasak province, Lao PDR. The 17th Regional Network on Asian Schistosomiasis and Other Helminth Zoonosis, Vientiane Capital, Lao PDR, October 24th–25th, 2017.
Ong KIC, Iwagami M, Hongvanthong B, Brey PT, Kano S, Jimba M. Towards malaria elimination in Lao PDR: a mixed methods study on health-seeking behaviors among different ethnic groups living in malaria endemic areas. The 3rd Annual the Future of Malaria Research Symposium, The Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA, November 3rd, 2017.
Iwagami M, Nakatsu M, Matsumoto-Takahashi M, Keomalaphet S, Khattignavong P, Soundala P, Lorphachan L, Hongvanthong B, Brey PT, Kano S. Analyses on k13 propeller gene mutations in Plasmodium falciparum revealed spread of artemisinin resistance in 5 Southern provinces of Lao PDR in 2015-2016, The 58th Annual Meeting of Japanese Society of Tropical Medicine, Hongo Campus, University of Tokyo, Tokyo, Japan, November 24th–26th, 2017.
Poster presentation:
Iwagami M, Nakatsu M, Keomalaphet S, Khattignavong P, Soundala P, Lorphachan L, Xangsayalath P, Pommelet V, Hongvanthong B, Brey PT, Kano S. Spread of artemisinin resistant Plasmodium falciparum in five Southern provinces of Lao PDR in 2015-2016, The 66th Annual Meeting of American Society of Tropical Medicine and Hygiene, Baltimore Convention Center, Baltimore, Maryland, USA, November 5th–9th, 2017.
Acknowledgements / Funding
We wish to thank Dr. Bouasy HONGVANTHONG, Director of the Center of Malariology, Parasitology and Entomology (CMPE), Ministry of Health, Lao PDR, and Project director of SATREPS for his kind support of this project. We thank Dr. Tiengkham PONGVONGSA, Deputy Director, Savannakhet Provincial Health Department, Lao PDR. We also thank the staff of the CMPE, the NIOPH, Provincial Health Offices, Provincial Hospitals, District Hospitals, and Health Centers, Lao Ministry of Health, for supporting our field surveys and training courses.
This work is supported by the SATREPS (Science and Technology Research Partnership for Sustainable Development) project from the Japan International Cooperation Agency (JICA) and the Japan Agency for Medical Research and Development (AMED).

1. Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. New England Journal of Medicine, 371: 411–423, 2014.
2. Ménard D, Khim N, Beghain J, Adegnika A, Alam M, Amodu O, et al. A Worldwide Map of Plasmodium falciparum Arteminisin Resistance. New England Journal of Medicine, 374:2453–2464, 2016.
3. Howes RE, Dewi M, Piel FB, Monteiro WM, Battle KE, Messina JP, et al. Spatial distribution of G6PD deficiency variants across malaria-endemic regions, Malaria Journal, 12: 418, 2013.
4. WHO Working Group. Glucose-6-phosphate dehydrogenase deficiency. Bulletin of the World Health Organization, 64: 601–611, 1989.
5. Yoon HJ, Ki M, Eom K, Yong TS, Chai JY, Min DY, et al. Risk factors for Opisthorchis viverrini and minute intestinal fluke infections in Lao PDR, 2009-2011. American Journal of Tropical Medicine and Hygiene, 91: 384-388. 2014.
6. Sohn WM, Yong TS, Eom KS, Min DY, Lee D, Jung BK., et al. Prevalence of Haplorchis taichui among humans and fish in Luang Prabang Province, Lao PDR. Acta Tropica, 136: 74–80, 2014.
7. Sayasone S, Utzinger J, Akkhavong K, Odermatt P. Repeated stool sampling and use of multiple techniques enhance the sensitivity of helminth diagnosis: a cross-sectional survey in southern Lao People’s Democratic Republic. Acta Tropica, 141(Pt B): 315–321, 2015.
8. Onsurathum S, Pinlaor P, Haonon O, Chaidee A, Charoensuk L, Intuyod K., et al. Effects of fermentation time and low temperature during the production process of Thai pickled fish (pla-som) on the viability and infectivity of Opisthorchis viverrini metacercariae. International Journal of Food Microbiology, 218: 1–5, 2016.
9. Vonghachack Y, Odermatt P, Taisayyavong K, Phounsavath S, Akkhavong K, Sayasone S. Transmission of Opisthorchis viverrini, Schistosoma mekongi and soil-transmitted helminthes on the Mekong Islands, Southern Lao PDR. Infectious Diseases of Poverty, 6: 131, 2017.

Lao National TV was interview scientists in Lao-Japan Laboratory








