5-Aminolevlinic acid asymptomatic malaria project
 Double-blind, parallel, randomized, placebo-controlled research to evaluate safety and efficacy of the 5-aminolevulinic phosphate (5-ALA PO4), sodium ferrous citrate (SFC) and zinc (Zn) with asymptomatic malaria parasite carriers
Double-blind, parallel, randomized, placebo-controlled research to evaluate safety and efficacy of the 5-aminolevulinic phosphate (5-ALA PO4), sodium ferrous citrate (SFC) and zinc (Zn) with asymptomatic malaria parasite carriers
Principal Investigator: Dr. Mayfong Mayxay, University of Health Sciences, Lao PDR
Members of the project:
Moritoshi IWAGAMI, Phonepadith
KHATTIGNAVONG, Sengdeuane KEOMALAPHET,
Phoyphaylinh PRASAYASITH, Pheovaly SOUNDALA,
Sonesimmaly SANNIKONE and Shigeyuki KANO
Background
Malaria morbidity and mortality have decreased in Lao PDR due to extensive efforts by the Lao Government and international organizations, such as World Health Organization (WHO), The Global Fund to Fight AIDS, Tuberculosis and Malaria. Recently, the Lao Ministry of Health and WHO have adopted a goal to achieve the elimination of malaria by 2030. However, several studies demonstrated that there were asymptomatic Plasmodium carriers in the malaria-endemic areas in Lao PDR. Most of them were adult population who had histories of malaria episodes and were engaged in forest-related occupations. Some studies also suggested that asymptomatic Plasmodium carriers can be a reservoir for the transmission of malaria by Anopheles mosquitos. However,  such people will never take any antimalarial medicines until they become symptomatic. In addition, most asymptomatic Plasmodium infections cannot be detected by standard diagnostic methods (microscopy and rapid diagnostic test: RDT) that are available in the endemic areas. Current malaria control and elimination strategy in Lao PDR is targeting for symptomatic malaria patients. Therefore, to accelerate the elimination of malaria in Lao PDR, a new effective strategy for targeting asymptomatic Plasmodium carriers is urgently needed in the endemic areas. 5-aminolevulinic acid (5-ALA), which is produced by neopharma Japan Co., Ltd., as a health food supplement commercially available in Japan, Philippines, Vietnam and UAE, is a natural precursor of heme in all animals. It is a non-protein amino acid synthesized in mitochondria and through the activity of cytochrome C oxidase is involved in the electron transport chain. It was found from pre-clinical studies that sodium ferrous citrate (SFC) enhanced Plasmodium falciparum-killing potency of 5-ALA and significantly inhibited the parasite growth both in vitro and in vivo [1, 2]. These novel findings may lead us to develop a new functional health supplement containing antimalarial activity using 5-ALA. Moreover, 5-ALA is being sold as a health food supplement, which has the functional claim “5-ALA supports to bring higher fasting blood glucose levels closer to normal” in Japan [3, 4]. In this study, we will evaluate the acceptability, safety and efficacy of 5-ALA phosphate (PO4) with SFC and Zn for asymptomatic Plasmodium carriers in malaria-endemic villages, Nong district, Savannakhet province, Lao PDR for one year. Efficacy of 5-ALA PO4 to Plasmodium infection will be examined by reduction of Plasmodium DNA positivity rate by PCR, compering to that of the Placebo group (only SFC and Zn). Since Zn deficiency is also a serious health problem in Lao PDR, participants of this study will take Zn as well to enhance a benefit for the study participants. In addition, to evaluate efficacy of 5-ALA PO4 to Plasmodium infection, we will evaluate the level of HbA1c, which is one of the markers of type 2 diabetes. It is reported that type 2 diabetes increases the risk of malaria infection [5]. Expected outcomes will contribute to malaria elimination and type 2 diabetes control in Lao PDR.
such people will never take any antimalarial medicines until they become symptomatic. In addition, most asymptomatic Plasmodium infections cannot be detected by standard diagnostic methods (microscopy and rapid diagnostic test: RDT) that are available in the endemic areas. Current malaria control and elimination strategy in Lao PDR is targeting for symptomatic malaria patients. Therefore, to accelerate the elimination of malaria in Lao PDR, a new effective strategy for targeting asymptomatic Plasmodium carriers is urgently needed in the endemic areas. 5-aminolevulinic acid (5-ALA), which is produced by neopharma Japan Co., Ltd., as a health food supplement commercially available in Japan, Philippines, Vietnam and UAE, is a natural precursor of heme in all animals. It is a non-protein amino acid synthesized in mitochondria and through the activity of cytochrome C oxidase is involved in the electron transport chain. It was found from pre-clinical studies that sodium ferrous citrate (SFC) enhanced Plasmodium falciparum-killing potency of 5-ALA and significantly inhibited the parasite growth both in vitro and in vivo [1, 2]. These novel findings may lead us to develop a new functional health supplement containing antimalarial activity using 5-ALA. Moreover, 5-ALA is being sold as a health food supplement, which has the functional claim “5-ALA supports to bring higher fasting blood glucose levels closer to normal” in Japan [3, 4]. In this study, we will evaluate the acceptability, safety and efficacy of 5-ALA phosphate (PO4) with SFC and Zn for asymptomatic Plasmodium carriers in malaria-endemic villages, Nong district, Savannakhet province, Lao PDR for one year. Efficacy of 5-ALA PO4 to Plasmodium infection will be examined by reduction of Plasmodium DNA positivity rate by PCR, compering to that of the Placebo group (only SFC and Zn). Since Zn deficiency is also a serious health problem in Lao PDR, participants of this study will take Zn as well to enhance a benefit for the study participants. In addition, to evaluate efficacy of 5-ALA PO4 to Plasmodium infection, we will evaluate the level of HbA1c, which is one of the markers of type 2 diabetes. It is reported that type 2 diabetes increases the risk of malaria infection [5]. Expected outcomes will contribute to malaria elimination and type 2 diabetes control in Lao PDR.
Objectives
• To assess the influence of 5-aminolevulinic acid phosphate (5-ALA PO4), sodium ferrous citrate (SFC) and zinc (Zn) to Plasmodium (Plasmodium DNA detected by PCR) in asymptomatic Plasmodium carriers.
• To assess the acceptability and safety of 5-ALA PO4 among Lao villagers who carry malaria parasites without symptoms as detected by PCR.
• To investigate the HbA1c level in asymptomatic malaria parasite carriers after administrations of 5-ALA PO4, SFC and Zn for daily usage.
The study period of the project
Two years (October 2019- September 2021)
Ethical approval
This study proposal was reviewed and approved by the Ethic Committee (No. 187), University of Health Sciences, Ministry of Health, Lao PDR on 26th June 2019 and the Institutional Review Board for Clinical Research (No. NCGM-G-003300-00), National Center for Global Health and Medicine (NCGM), Japan on 27th September 2019. Permission of importation of 5-ALA PO4 (No. 9330) was also obtained from the Department of Food and Drug, Ministry of Health, Lao PDR on 20th September 2019.
Methodology
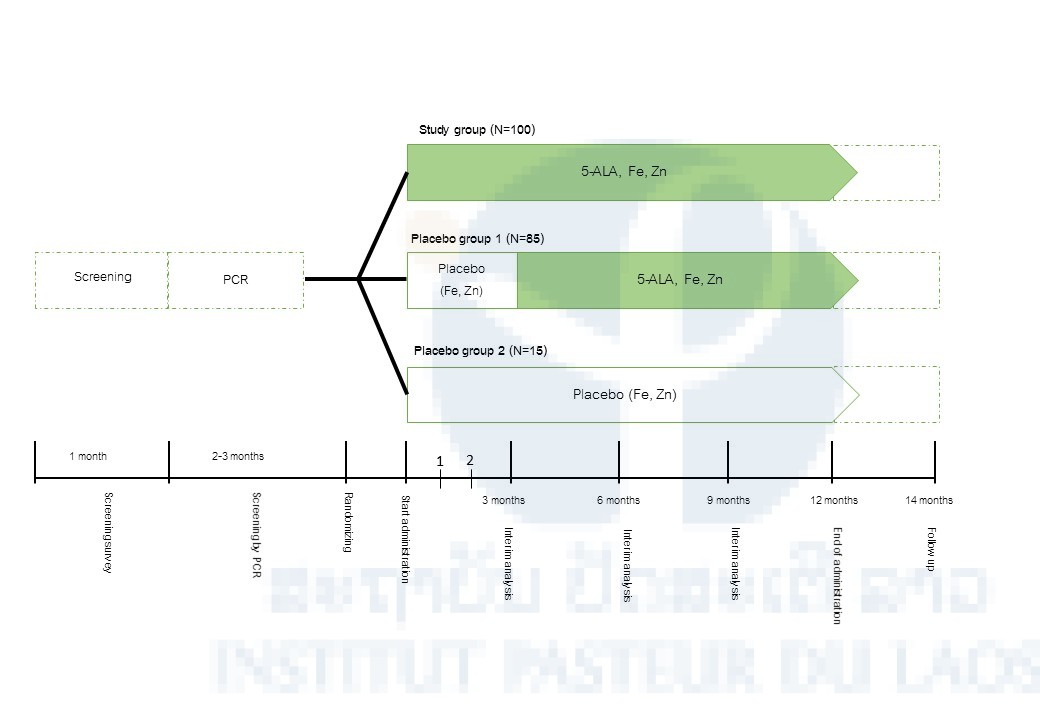
After having provided informed consent, potential participants will be enrolled in a screening during which all inclusion/exclusion criteria, including laboratory assessments, will be checked for eligibility. If full eligibility is confirmed, the participants will be randomized to three arms: Arm 1: 5-ALA PO4 25 mg/ day + SFC 28.7 mg + Zinc 10 mg (12 months), Arm 2: Placebo + SFC 28.7 mg + Zinc 10 mg (3, 6 or 9 months*) and then 5-ALA PO4 25 mg/day + SFC 28.7 mg + Zinc 10 mg (3, 6 or 9 months) or Arm 3: SFC 28.7 mg + Zinc 10 mg (12 months). Interim Analysis will be carried out at 3, 6 or 9 months (Figure 1).
*When the statistical difference of Plasmodium DNA positivity rate between Arm 1 and Arm 2 is observed at 3 months, Arm 2 participants will take 5-ALA PO4 25 mg/day + SFC 28.7 mg + Zinc 10 mg from 3 months through the end of the study period. If no statistical difference between the 2 groups is observed at 3 months and the statistical difference is observed at 6 months, Arm 2 participants will take 5-ALA PO4 25 mg/day + SFC 28.7 mg + Zinc 10 mg from 6 months through the end of the study period. If no statistical difference between the 2 groups is observed at 6 months and the statistical difference is observed at 9 months, Arm 2 participants will take 5-ALA PO4 25 mg/day + SFC 28.7 mg + Zinc 10 mg from 9 months through the end of the study period. If no statistical difference between the 2 groups is observed at 9 months, Arm 2 participants will take only SFC 28.7 mg + Zinc 10 mg for 12 months (entire the study period).
Clearance of Plasmodium DNA [Time Frame: 1, 2, 3, 6, 9 and 12 months] Defined as the positive rate of blood-stage of Plasmodium among participants confirmed by PCR and HbA1c defined as the actual value from blood confirmed by handy HbA1c monitoring device within all-time periods are provided in Table 1. Blood sample (maximum 800μL per sampling) will be collected using lancet, syringe, needle and preserved on filter paper.
Follow up surveys will be conducted at 2 months later at the end of the administration of 5-ALA or Placebo. In the follow-up survey, a blood sample will be collected and examined by PCR for checking Plasmodium DNA.
Current situation (as of 31st October 2019)
The first field survey for the screening of asymptomatic Plasmodium carriers started on 20th October 2019 (until 14th November 2019). More than 1,100 blood samples were collected from adult participants (age: 18-65 years old) in malaria-endemic villages, Nong district, Savannakhet province. Those who had any signs and symptoms of malaria and pregnant ladies were excluded from the screening. PCR screening for detecting Plasmodium infection will be performed at IPL and NCGM. Administration of 5-ALA PO4 to asymptomatic Plasmodium carriers will be performed by the team of the University of Health Sciences leaded by Dr. Mayfong Mayxay. The roles of IPL in this project is laboratory analyses (DNA detection by PCR or LAMP methods) and support the field works.
Financial support
This work is financially supported by neopharma Japan Co. Ltd.
References
1. Komatsuya K, Hata M, Balogun EO, Hikosaka K, Suzuki S, Takahashi K, Tanaka T, Nakajima M, Ogura S, Sato S, Kita K. Synergy of ferrous ion on 5-aminolevulinic acid-mediated growth inhibition of Plasmodium falciparum. Journal of Biochemistry, 154(6): 501-504, 2013.
2. Suzuki S, Hikosaka K, Balogun EO, Komatsuya K, Niikura M, Kobayashi F, Takahashi K, Tanaka T, Nakajima M, Kita K. In vivo curative and protective potential of orally administered 5-aminolevulinic acid plus ferrous ion against malaria. Antimicrobial Agents and Chemotherapy, 59(11): 6960-6967, 2015.
3. Higashikawa F, Noda M, Awaya T, Tanaka T, Sugiyama M. 5-aminolevulinic acid, a precursor of heme, reduces both fasting and postprandial glucose levels in mildly hyperglycemic subjects. Nutrition, 29(7-8): 1030-10306, 2013.
4. Al-Saber F, Aldosari W, Alselaiti M, Khalfan H, Kaladari A, Khan G, Harb G, Rehani R, Kudo S, Koda A, Tanaka T, Nakajima M, Darwish A. The Safety and Tolerability of 5-Aminolevulinic Acid Phosphate with Sodium Ferrous Citrate in Patients with Type 2 Diabetes Mellitus in Bahrain. Journal of Diabetes Research, 2016: 8294805, 2016.
5. Danquah I, Bedu-Addo G, Mockenhaupt FP. Type 2 diabetes mellitus and increased risk for malaria infection. Emerging Infectious Diseases, 16(10): 1601-1604, 2010.
Scientific communications
Publications:
Ong KIC, Iwagami M, Araki H, Khattignavong P, Soundala P, Keomalaphet S, Prasayasith P, Lorpachan L, Xangsayalath P, Pongvongsa T, Hongvanthong B, Brey PT, Kano S, Jimba M. Prevalence of G6PD Viangchan variant in malaria-endemic areas in Lao PDR: an implication for malaria elimination by 2030, Malaria Journal, 18: 75, 2019.
Vilay P, Nonaka D, Senamonty P, Lao M, Iwagami M, Kobayashi J, Hernandez PM, Phrasisombath K, Kounnavong S, Hongvanthong B, Brey PT, Kano S. Malaria prevalence, knowledge, perception, preventive and treatment behavior among military in Champasack and Attapeu provinces, Lao PDR: a mixed-methods study, Tropical Medicine and Health, 47: 11, 2019.
Pongvongsa T, Nonaka D, Iwagami M, Soundala P, Khattignavong P, Xangsayarath P, Nishimoto F, Kobayashi J, Hongvanthong B, Brey P, Kano S. Malaria among foreign migrant workers in Savannakhet Province, Lao PDR: a cross-sectional study, Tropical Medicine and Health, 47: 10, 2019.
Iwagami M, Nakatsu M, Khattignavong P, Soundala P, Keomalaphet S, Lorpachan L, Xangsayalath P, Matsumoto-Takahashi E, Pommelet V, Hongvanthong B, Brey PT, Kano S. Heterogeneous distribution of K13 mutations in Plasmodium falciparum in Lao PDR. Malaria Journal, 17: 483, 2018.
Takahashi E, Nonaka D, Iwagami M, Vilay P, Chanthakoumane K, Kobayashi J, Pongvongsa T, Kounnavong S, Hongvanthong B, Brey PT, Kano S. Patients’ adherence to artemisinin-based combination therapy and healthcare workers’ perception and practice in Savannakhet province, Lao PDR, Tropical Medicine and Health, 46: 44, 2018.
Iwagami M, Tangpukdee N, Wilairatana P, Krudsood S, Dao LD, Nakazawa S, Sinuon M, Socheat D, Yasuoka J, Jimba M, Watanabe H, Kobayashi J, Toma H, Vanisaveth V, Hongvanthong V, Brey PT, Kano S. Pfcrt genotypes and microsatellite DNA polymorphisms flanking the gene on Plasmodium falciparum suggest different chloroquine selective pressure among populations in the Greater Mekong Subregion, Parasitology International, 67: 816- 823, 2018.
Vincent JP, Komaki‑Yasuda K, Iwagami M, Kawai S, Kano S. Combination of PURE‑DNA extraction and LAMP‑DNA amplification methods for accurate malaria diagnosis on dried blood spots, Malaria Journal, 17: 373, 2018.
Iwagami M, Nakatsu M, Khattignavong P, Soundala P, Lorphachan L, Keomalaphet S, Xangsayalath P, Kawai S, Hongvanthong B, Brey PT, Kano S. First confirmed case of human infection with Plasmodium knowlesi in Lao PDR, PLOS Neglected Tropical Diseases, 12(3): e0006244, 2018.
Araki H, Ken Ong KIC, Lorphachan L, Soundala P, Iwagami M, Shibanuma A, Hongvanthong B, Brey PT, Kano S, Jimba M. Mothers’ Opisthorchis viverrini infection status and raw fish dish consumption in Lao People’s Democratic Republic: determinants of child infection status, Tropical Medicine and Health, 46: 29, 2018.
Iwagami M, Keomalaphet S, Khattignavong P, Soundala P, Lorphachan L, Matsumoto-Takahashi E, Strobel M, Reinharz D, Phommasansack M, Hongvanthong B, Brey PT, Kano S. The detection of cryptic Plasmodium infection among villagers in Attapeu province, Lao PDR. PLOS Neglected Tropical Diseases, 11(12): e0006148, 2017.
Inthavong N, Nonaka D, Kounnavong S, Iwagami M, Phommala S, Kobayashi J, Hongvanthong B, Pongvongsa T, Brey PT, Kano S. Individual and household factors associated with incidences of village malaria in Xepon district, Savannakhet province, Lao PDR. Tropical Medicine and Health, 45: 36, 2017.
Ong KIC, Kosugi H, Thoeun S, Araki H, Thandar MM, Iwagami M, Hongvanthong B, Brey PT, Kano S, Jimba M. Systematic review of the clinical manifestations of glucose-6-phosphate dehydrogenase deficiency in the Greater Mekong Subregion: implications for malaria elimination and beyond. BMJ Global Health, 2: e000415, 2017.
Ménard D, Khim N, Beghain J, Adegnika AA, Shafiul- Alam M, Amodu O, Rahim-Awab G, Barnadas C, Berry A, Boum Y, Bustos MD, Cao J, Chen JH, Collet L, Cui L, Thakur GD, Dieye A, Djallé D, Dorkenoo MA, Eboumbou-Moukoko CE, Espino FE, Fandeur T, Ferreirada- Cruz MF, Fola AA, Fuehrer HP, Hassan AM, Herrera S, Hongvanthong B, Houzé S, Ibrahim ML, Jahirul- Karim M, Jiang L, Kano S, Ali-Khan W, Khanthavong M, Kremsner PG, Lacerda M, Leang R, Leelawong M, Li M, Lin K, Mazarati JB, Ménard S, Morlais I, Muhindo- Mavoko H, Musset L, Na-Bangchang K, Nambozi M, Niaré K, Noedl H, Ouédraogo JB, Pillai DR, Pradines B, Quang-Phuc B, Ramharter M, Randrianarivelojosia M, Sattabongkot J, Sheikh-Omar A, Silué KD, Sirima SB, Sutherland C, Syafruddin D, Tahar R, Tang LH, Touré OA, Tshibangu-wa-Tshibangu P, Vigan-Womas I, Warsame M, Wini L, Zakeri S, Kim S, Eam R, Berne L, Khean C, Chy S, Ken M, Loch K, Canier L, Duru V, Legrand E, Barale JC, Stokes B, Straimer J, Witkowski B, Fidock DA, Rogier C, Ringwald P, Ariey F, Mercereau- Puijalon O; KARMA Consortium. A Worldwide Map of Plasmodium falciparum Artemisinin Resistance. New England Journal of Medicine. 374: 2453–2464, 2016.
Pongvongsa T, Nonaka D, Iwagami M, Nakatsu M, Phongmany P, Nishimoto F, Kobayashi J, Hongvanthong B, Brey PT, Moji K, Mita T, Kano S. Household clustering of asymptomatic malaria infections in Xepon district, Savannakhet province, Lao PDR. Malaria Journal. 15: 508, 2016.
Oral presentations:
1. Moritoshi Iwagami, Phonepadith Khattignavong, Mayfong Mayxay, Paul T. Brey, Shigeyuki Kano, Development of new clinical research beyond the SATREPS project, 13th National Health Research Forum 2019, Vientiane, Done Chanh Palace Hotel, Lao PDR, 15th-17th October 2019.
2. Masami Nakatsu, Moritoshi Iwagami, Sengdeuane Keomalaphet, Phonepadith Khattignavong, Pheovaly Soundala, Lavy Lorphachan, Bouasy Hongvanthong, Brey Paul, Shigeyuki Kano, Analysis of artemisinin-resistant gene of Plasmodium falciparum in Phongsaly, the northern-most province in Laos, The 88th Annual Meeting of Japanese Society of Parasitology, Nagasaki University, Nagasaki city, Japan, 15th -16th, March 2019.
3. Takashi Kumagai, Moritoshi Iwagami, Emilie Matsumoto-Takahashi, Keomalaphet Sengdeuane, Phonepadith Khattignavong, Lavy Lorphachan, Pheovaly Soundala, Bouasy Hongvanthong, Kei Oyoshi, Yousei Mizukami, Yoshinobu Sasaki, Nobuo Ohta, Paul T. Brey, Shigeyuki Kano, Risk mapping of Schistosomiasis mekongi using LAMP method and earth observation satellite data, The 88th Annual Meeting of Japanese Society of Parasitology, Nagasaki University, Nagasaki city, Japan, 15th -16th, March 2019
4. Emilie Matsumoto-Takahashi, Takashi Kumagai, Moritoshi Iwagami, Yoshinobu Sasaki, Yousei Mizukami, Kei Oyoshi, Shigeyuki Kano, Impact of precipitation on schistosomiasis mekongi in Lao PDR: Spatial epidemiology using earth observation satellite data, The 88th Annual Meeting of Japanese Society of Parasitology, Nagasaki University, Nagasaki city, Japan, 15th -16th, March 2019
Poster presentation:
1. Ken Ing Cherng Ong, Phonepadith Khattignavong, Sengdeuane Keomalaphet, Moritoshi Iwagami, Bouasy Hongvanthong, Paul T. Brey, Shigeyuki Kano, Masamine Jimba. Listening to the voices of the vulnerable: a mixed-methods study on health-seeking behaviors in a malaria-endemic district in Lao People’s Democratic Republic. The 68th Annual Meeting of American Society of Tropical Medicine and Hygiene, Gaylord National Resort and Convention Center National Harbor, Maryland, USA, November 21st -24th, 2019.
Table 1. List of dried blood samples on filter paper collected from malaria patients and suspected patients in five southern provinces and one northernmost province
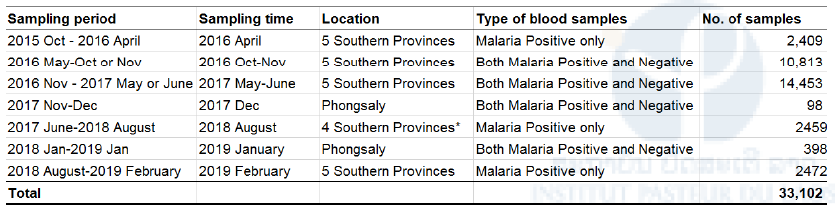
Malaria diagnoses was performed by microscopy or RDT. *Blood samples were not collected from Attapeu because of flooding in July 2018.
Table 2. Summary of Plasmodium species in five Southern Provinces 2015-2017
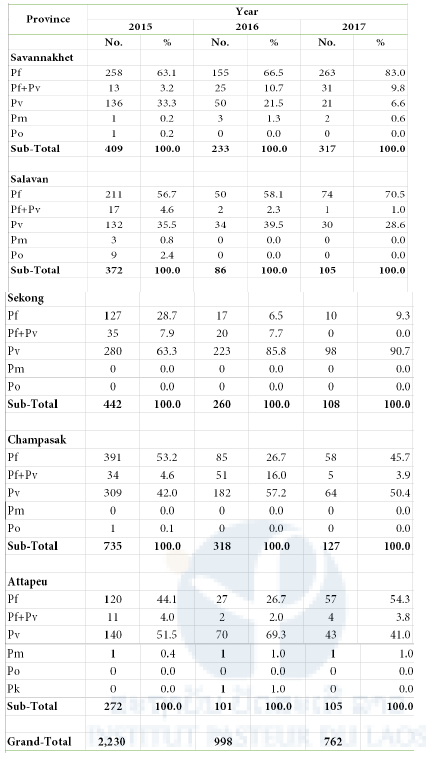
Pf: P. falciparum; Pv: P. vivax; Pm: P. malariae; Po: P. ovale; Pk: P. knowlesi
Sampling period:
2015: October 2015 – April 2016
2016: May – October or November 2016
2017: November 2016 – May or June 2017
Table 3. Prevalence of artemisinin-resistant mutation in K13 gene in P. falciparum in five Southern Provinces 2015-2017
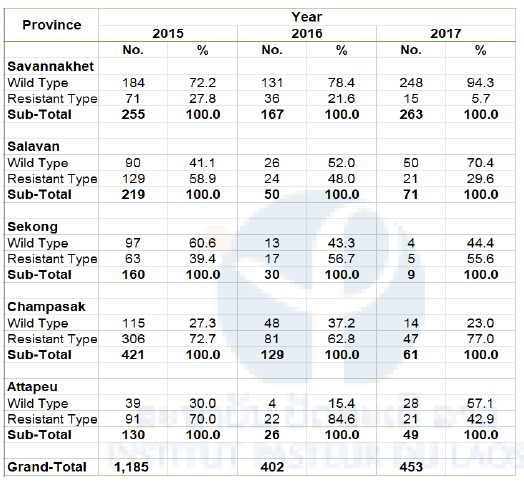
Sampling period
2015: October 2015 – April 2016
2016: May-October or November 2016
2017: November 2016 – May or June 2017
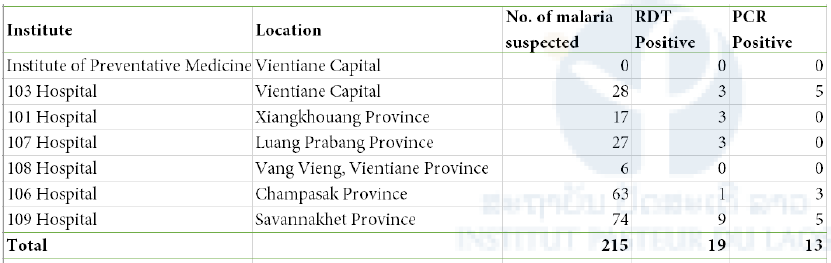
Table 4. Summary of blood sample testing by malaria RDT and PCR