5-Aminolevlinic acid asymptomatic malaria project

Double blind, parallel, randomized, placebocontrolled
research to evaluate the safety and efficacy of
the 5-aminolevulinic phosphate (5-ALA PO4), sodium
ferrous citrate (SFC) and zinc (Zn) with asymptomatic
malaria parasite carriers

Principal Investigator in Lao PDR:
Dr. Mayfong MAYXAY, University of Health Sciences
(UHS), Lao PDR
Principal Investigator in Japan:
Dr. Shigeyuki KANO, IPL and National Center for Global
Health and Medicine (NCGM), Japan
Staff members in Parasitology Lab:
Dr. Moritoshi IWAGAMI, Dr. Phonepadith
KHATTIGNAVONG, Dr. Sengdeuane KEOMALAPHET, Dr. Phoyphaylinh PRASAYASITH, Ms. Pheovaly SOUNDALA, Ms. Sonesimmaly SANNIKONE, and Mr.Takashi SEKINE
Background
Recently, the Lao Ministry of Health adopted a goal to achieve the elimination of malaria by 2030. However, several studies demonstrated that there were asymptomatic Plasmodium carriers (hidden malaria, parasite reservoir) in the malaria endemic areas in Lao PDR [1-3]. Most of them were adult population who had histories of malaria episodes and were engaged on forest related occupations [1]. Some studies also suggested that asymptomatic Plasmodium carriers can be a reservoir for transmission of malaria by Anopheles mosquitos. However, such people will never take any antimalarial medicines until they become symptomatic. In addition, most asymptomatic Plasmodium infections cannot be detected by standard diagnostic methods (microscopy and RDT) that are available in the endemic areas. The current malaria control and elimination strategy in Lao PDR is targeting only symptomatic malaria patients. Therefore, to accelerate the elimination of malaria in Lao PDR, a new effective strategy for targeting asymptomatic Plasmodium carriers is urgently needed in the endemic areas.
5-aminolevulinic acid (5-ALA) which is produced by neopharma Japan Co., Ltd. as a health food supplement and commercially available in Japan, Philippines, Vietnam and UAE, is a natural precursor of heme in all animals. It is a non-protein amino acid synthesized in mitochondria and through the activity of cytochrome c oxidase is involved in the electron transport chain. It was found in pre-clinical studies that sodium ferrous citrate (SFC) ) enhanced P. falciparum-killing potency of 5-ALA and significantly inhibited the parasite growth both in vitro and in vivo [4,5]. These novel findings may lead us to develop a new functional health supplement containing antimalarial activity using 5-ALA. In addition, 5-ALA is being sold as a health food supplement, which has the functional claim “5-ALA supports to bring higher fasting blood glucose levels closer to normal” in Japan [6,7].
In this study, we will evaluate the acceptability, safety and efficacy of 5-ALA phosphate (PO4) with SFC and Zn for asymptomatic Plasmodium carriers in malaria endemic villages, Nong and Sepon districts, Savannakhet province, Lao PDR for one year. The efficacy of 5-ALA PO4 to Plasmodium infection will be examined by reduction of Plasmodium DNA positivity rate by PCR, compering to that of Placebo group (only SFC and Zn). Since Zn deficiency is also a serious health problem in Lao PDR, participants of this study will take Zn as well to enhance a benefit for the study participants. In addition, to evaluate the efficacy of 5-ALA PO4 to Plasmodium infection, we will also evaluate the level of HbA1c, which is one of the markers of type 2 diabetes. It is also reported that type 2 diabetes increases the risk for malaria infection [8]. Expected outcomes will contribute to malaria elimination as well as type 2 diabetes control in Lao PDR.
Objectives
+To assess the influence of 5-ALA PO4, SFC and Zn to Plasmodium (Plasmodium DNA detected by PCR) in asymptomatic Plasmodium carriers.
+To assess the acceptability and safety of 5-ALA PO4 among Lao villagers who carry malaria parasites without symptoms as detected by PCR.
+To investigate the HbA1c level in asymptomatic malaria parasite carriers after administrations of 5-ALA PO4, SFC and Zn for daily usage.
The study period of the project
Two years (October 2019- September 2021)
Ethical approval
This study proposal was reviewed and approved by the Ethics Committee (No. 187), University of Health Sciences, Ministry of Health, Lao PDR on 26th June 2019 and the Institutional Review Board for Clinical Research (No. NCGM-G-003300-00), National Center for Global Health and Medicine (NCGM), Japan on 27th September 2019. Permission of importation of 5-ALA PO4 (No. 9330) was also obtained from the Department of Food and Drug, Ministry of Health, Lao PDR on 20th September 2019.
Methodology
After having provided informed consent, potential participants (age: 18-65 years old) will be enrolled into a screening during which all inclusion/exclusion criteria, including laboratory assessments, will be checked for eligibility. Those who had any signs and symptoms of malaria, malaria RDT positive, and pregnant ladies were excluded from the screening. PCR screening for detecting Plasmodium infection will be performed at IPL and NCGM. If full eligibility is confirmed, the participants will be randomized to three arms: Arm 1: 5-ALA PO4 25 mg/day + SFC 28.7 mg + Zinc 10 mg (12 months), Arm 2: Placebo + SFC 28.7 mg + Zinc 10 mg (3, 6 or 9 months*) and then 5-ALA PO4 25 mg/day + SFC 28.7 mg + Zinc 10 mg (3, 6 or 9 months) or Arm 3: SFC 28.7 mg + Zinc 10 mg (12 months). Interim Analysis will be carried out at 3, 6, or 9 months.
*When the statistical difference of Plasmodium DNA positivity rate between Arm 1 and Arm 2 is observed at 3 months, Arm 2 participants will take 5-ALA PO4 25 mg/day + SFC 28.7 mg + Zinc 10 mg from 3 months through the end of the study period. If no statistical difference between the 2 groups is observed at 3 months and the statistical difference is observed at 6 months, Arm 2 participants will take 5-ALA PO4 25 mg/day + SFC 28.7 mg + Zinc 10 mg from 6 months through the end of the study period. If no statistical difference between the 2 groups is observed at 6 months and the statistical difference is observed at 9 months, Arm 2 participants will take 5-ALA PO4 25 mg/day + SFC 28.7 mg + Zinc 10 mg from 9 months through the end of the study period. If no statistical difference between the 2 groups is observed at 9 months, Arm 2 participants will take only SFC 28.7 mg + Zinc 10 mg for 12 months (entire study period).
Clearance of Plasmodium DNA [Time Frame: 1, 2, 3, 6, 9 and 12 months] Defined as the positive rate of blood-stage of Plasmodium among participants confirmed by PCR and HbA1c defined as the actual value from blood confirmed by handy HbA1c monitoring device (A1C Now®, PTS Diagnostic, USA). Blood samples (maximum 800μL per sampling) will be collected using lancet or syringe and needle and preserved on filter paper. Follow up survey will be conducted 2 months later at the end of the administration of 5-ALA PO4 or Placebo. In the follow-up survey, a blood sample will be collected and examined by PCR for checking Plasmodium DNA.
Screening of asymptomatic Plasmodium carriers
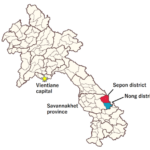
Three field surveys for the screening of asymptomatic Plasmodium carriers were conducted in Nong district (20th October-14th November 2019), in Sepon district (15th-28th December 2019) and in Nong and Sepon districts (4th-24th February 2020), Savannakhet province (Figure 1). All the surveys were conducted with a team consisted of IPL, UHS, CMPE, Savannakhet Provincial Health Department, Nong and Sepon District Health Offices, Health Centers in collaboration with village chiefs and village health volunteers.
In the first field survey in Nong district, 2,716 villagers in 32 villages participated in the survey and 13 of them were tested positive for P. falciparum and six were tested positive for P. vivax by RDT (Malaria Ag P.f/P.v, Standard Diagnostics Inc., Republic of Korea) (Table 4). This RDT is able to detect histidine-rich protein-II (HRP-II) in P. falciparum and lactate dehydrogenase (pLDH) in P. vivax that is commonly used for malaria diagnosis in Lao PDR.
In the second field survey in Sepon district, 1,353 villagers in 28 villages participated in the survey and eight of them were tested positive for P. falciparum and one was tested positive for P. falciparum and P. vivax (mixed infection) by RDT (Table 4). In this survey, we used two types of malaria RDT. The one is the standard RDT (Malaria Ag P.f/P.v) and the other is Malaria Ag P.f/P.f/P.v (Standard Diagnostics Inc., Republic of Korea). The latter (Malaria Ag P.f/P.f/P.v) is able to detect both HRP-II and pLDH in P. falciparum as well as pLDH in P. vivax, which means this RDT can detect an infection of HRP-II deleted P. falciparum. We used 1,025 tests of Malaria Ag P.f/P.f/P.v in this second survey. In fact, we found a case of suspected Pf HRP-II deletion in P. falciparum in Keang Nai village. To validate Pf HRP-II deletion, PCR confirmation will be performed by using specific primer sets for pfhrp-II and the other P. falciparum genes [9].
In the third field survey in Nong and Sepon districts, 2,260 villagers in 35 villages (Nong: n=1,647 in 23 villages, Sepon: n=613 in 12 villages) participated in the survey. In Nong district, four of them were tested positive for P. vivax and one was tested positive for P. falciparum and P. vivax (mixed infection) by RDT (Table 4). In Sepon district, only one of them was tested positive for P. vivax by RDT. In this survey, we used the standard RDT (Malaria Ag P.f/P.v). All the study participants (malaria patients) who were diagnosed positive for malaria by the RDTs were treated with Coartem (artemetherlumefantrine) at health centers free of charge, which is a standard treatment regimen for uncomplicated malaria in Lao PDR. Malaria treatment in Lao PDR is fully supported by the Global Fund.
Malaria screening PCR analysis was performed using primer sets previously designed [10] at IPL and NCGM using the RDT negative samples because of the RDT positive means malaria patients who are not targeted for this study. One of the inclusion criteria in this study is malaria RDT negative and malaria PCR positive. The result of the PCR analysis is summarized in Table 4 and Figure 2. In the first survey, 41 were tested positive by the PCR. In the second survey, only four were tested positive by the PCR. In the third survey, 21 were tested positive by the PCR. Therefore, a total of 66 villagers (asymptomatic malaria carriers) were eligible candidates for recruiting for the 5-ALA supplement study.
Administration of the supplement
To avoid time lag between the screening survey and the supplement administration, the 5-ALA PO4 supplement or placebo was administered to the eligible participants when the PCR analysis was finished for each survey. The supplement administration was initiated in February, March and June for group 1 (participants in the 1st screening survey), group 2 (participants in the 2nd screening survey) and group 3 (participants in the 3rd screening survey), respectively (Table 5). In group 1, one eligible candidate refused to participate in the study for an unknown reason (Figure 2). In group 3, one eligible candidate was excluded from the study because according to the local healthcare staff and village chief, the candidate was an illegal drug user and often disappeared from his village (Figure 2).
One tablet of the supplement or placebo is taken every day at any time by the study participant (subject). Adherence to the supplement or placebo is monitored by several levels of the local people. Chief of village or village health volunteer monitors the administration daily by using check sheet and counting remaining tablets. Health Center staff also monitors the administration and health condition of the participants weekly at the first three months, then once every two weeks on and after four months, by visiting the study participants. District Health Office staff also monitors Health Center staff every week by telephone or visiting the Health Centers. IPL staff also monitors the district staff and the Health Center staff by telephone or social networking service every week or once every two weeks.
The follow-up of the participants was conducted by the follow-up team (IPL, UHS, CMPE, Provincial Health Department staff, District Health Office staff, and Health Center staff) according to the follow-up schedule in Table 5. In the follow-up, basic health checks (body temperature, health consultation, pregnancy test), HbA1c test, and dried blood sample collection on filter paper for PCR were conducted. HbA1c test was performed by using A1CNow® (PTS Diagnostics, USA).
Impact of the lockdown due to COVID-19 for this study
Due to the lockdown of COVID-19 in Lao PDR, the follow-up in April and May 2020 was conducted by the Health Center staff. Although it was prohibited to travel between provinces in the country in April due to the strict lockdown, IPL obtained travel permission from the Lao Ministry of Health and IPL driver, Mr. Hongnakhone XAYASING managed to deliver the supplements and consumables to Nong District Health Office. In fact, he could not reach the Nong District Health Office but could reach a gate to Nong district. Then, he handed over the supplements and consumables to the Health Center staff at the gate and the Health Center staff delivered them to the Nong District Health Office. Unfortunately, the HbA1c test was not able to be performed in April and May because it is difficult for the local healthcare staff to use the test kit which is highly sensitive to temperature (18oC-28oC). In addition, the test kit has to be stored in the refrigerator, which is not available in some Health Centers.
Financial support
This work is financially supported by neopharma Japan Co., Ltd.
References
1. Iwagami M, Keomalaphet S, Khattignavong P, Soundala P, Lorphachan L, Matsumoto-Takahashi E, Strobel M, Reinharz D, Phommasansack M, Hongvanthong B, Brey PT, Kano S. The detection of cryptic Plasmodium infection among villagers in Attapeu province, Lao PDR. PLoS Neglected Tropical Diseases, 11(12): e0006148, 2017.
2. Vilay P, Nonaka D, Senamonty P, Lao M, Iwagami M, Kobayashi J, Hernandez PM, Phrasisombath K, Kounnavong S, Hongvanthong B, Brey PT, Kano S. Malaria prevalence, knowledge, perception, preventive and treatment behavior among military in Champasak and Attapeu provinces, Lao PDR: a mixed methods study. Tropical Medicine and Health, 47:11, 2019.
3. Pongvongsa T, Nonaka D, Iwagami M, Nakatsu M, Phongmany P, Nishimoto F, Kobayashi J, Hongvanthong B, Brey PT, Moji K, Mita T, Kano S. Household clustering of asymptomatic malaria infections in Xepon district, Savannakhet province, Lao PDR. Malaria Journal, 15(1): 508, 2016.
4. Komatsuya K, Hata M, Balogun EO, Hikosaka K, Suzuki S, Takahashi K, Tanaka T, Nakajima M, Ogura S, Sato S, Kita K. Synergy of ferrous ion on 5-aminolevulinic acidmediated growth inhibition of Plasmodium falciparum. Journal of Biochemistry, 154(6): 501-504, 2013.
5. Suzuki S, Hikosaka K, Balogun EO, Komatsuya K, Niikura M, Kobayashi F, Takahashi K, Tanaka T, Nakajima M, Kita K. In vivo curative and protective potential of orally administered 5-aminolevulinic acid plus ferrous ion against malaria. Antimicrobial Agents and Chemotherapy, 59(11): 6960-6967, 2015.
6. Higashikawa F, Noda M, Awaya T, Tanaka T, Sugiyama M. 5-aminolevulinic acid, a precursor of heme, reduces both fasting and postprandial glucose levels in mildly hyperglycemic subjects. Nutrition, 29(7-8): 1030-10306, 2013.
7. Al-Saber F, Aldosari W, Alselaiti M, Khalfan H, Kaladari A, Khan G, Harb G, Rehani R, Kudo S, Koda A, Tanaka T, Nakajima M, Darwish A. The Safety and Tolerability of 5-Aminolevulinic Acid Phosphate with Sodium Ferrous Citrate in Patients with Type 2 Diabetes Mellitus in Bahrain. Journal of Diabetes Research, 2016: 8294805, 2016.
8. Danquah I, Bedu-Addo G, Mockenhaupt FP. Type 2 diabetes mellitus and increased risk for malaria infection. Emerging Infectious Diseases, 16(10): 1601-1604, 2010.
9. Global Malaria Programme, False-negative RDT results and P. falciparum histidine-rich protein 2/3 gene deletions, World Health Organization, 2016 (Rev. 2019).
10. Iwagami M, Nakatsu M, Khattignavong P, Soundala P, Keomalaphet S, Lorpachan L, Xangsayalath P, Matsumoto‑Takahashi E, Pommelet V, Hongvanthong V, Brey PT, Kano K. Heterogeneous distribution of k13 mutations in Plasmodium falciparum in Laos. Malaria Journal, 17: 483, 2018.
Scientific communications
Oral presentation:
1. Moritoshi Iwagami, Masami Nakatsu, Sengdeuane Keomalaphet, Phonepadith Khattignavong, Pheovaly Soundala, Bouasy Hongvanthong, Paul Brey, Shigeyuki Kano. Molecular surveillance of artemisinin resistant Plasmodium falciparum in Lao PDR during 2015-2017. Korean Society of Parasitology and Tropical Medicine conference, October 22nd, 2020 (Online meeting)
Poster presentation:
1. Moritoshi Iwagami, Masami Nakatsu, Sengdeuane Keomalaphet, Phonepadith Khattignavong, Pheovaly Soundala, Bouasy Hongvanthong, Paul Brey, Shigeyuki Kano. Prevalence and frequency of artemisinin resistant Plasmodium falciparum in Laos during 2015-2017. The 69th Annual Meeting of American Society of Tropical Medicine and Hygiene, November 15th -19th, 2020 (Online meeting).
Tables
Table 1. Summary of blood sample testing by malaria RDT and PCR in 2019
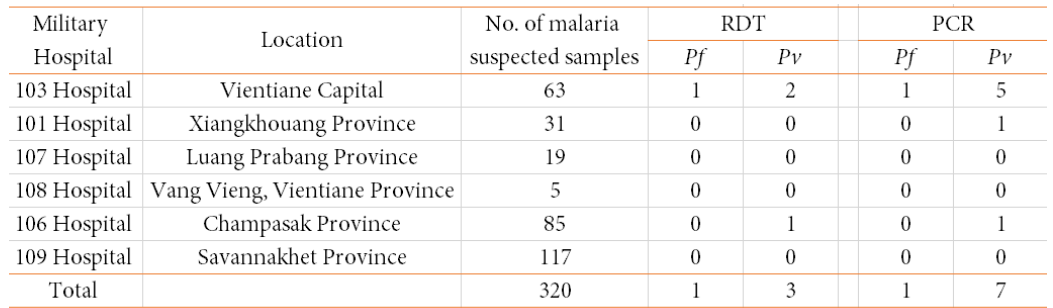
Pf: P. falciparum; Pv. P. vivax
Table 2. Summary of blood sample testing by malaria RDT and PCR in 202
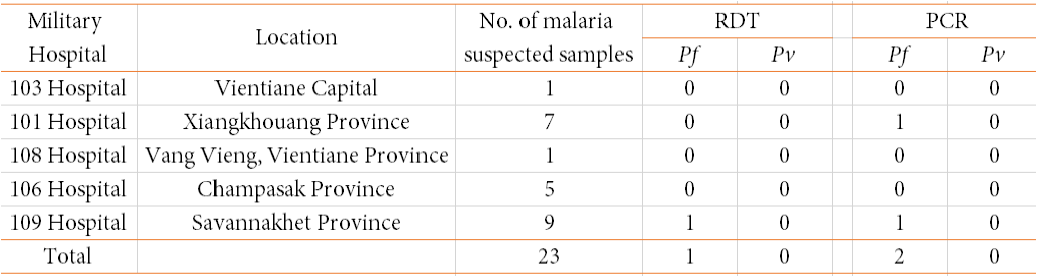
Pf: P. falciparum; Pv. P. vivax
Table 3. Number of TES samples 2019-2020
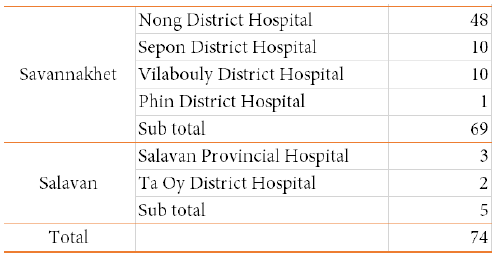
Table 4. Summary of the screening survey for the 5-ALA asymptomatic malaria study in Savannakhet province
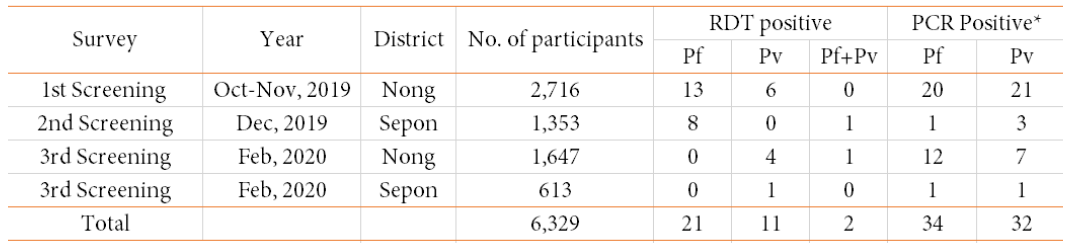
* PCR positive but RDT negative
Table 5. Follow-up schedule of the 5-ALA asymptomatic malaria study in 2020
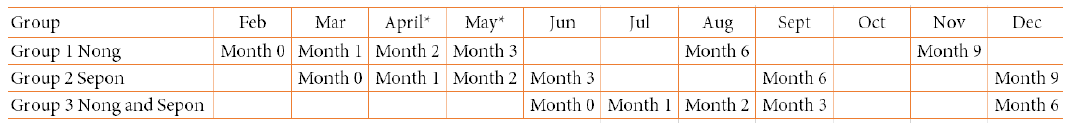
* HbA1c data was not obtained due to the lockdown of COVID-19 pandemic. * Dried blood samples on filter paper were collected by the local staff.
Table 6. Follow-up schedule of the 5-ALA asymptomatic malaria study in 2021
Figure Legend
Figure 1. Study sites of the 5-ALA asymptomatic malaria study
Figure 2. Number of subjects of the 5-ALA asymptomatic malaria study
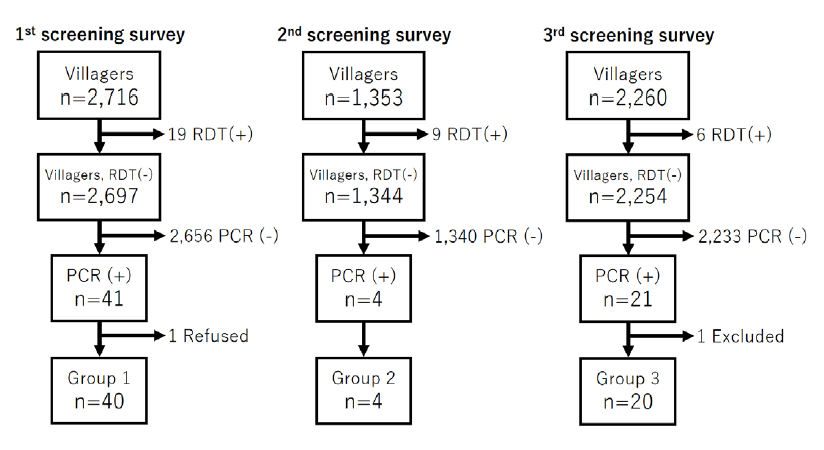
Villagers who were malaria RDT positive and malaria PCR negative were excluded from the study. One eligible candidate in the 1st screening survey was refused to participate in this study. Another eligible candidate in the 3rd screening survey was excluded from this study because the one was an illegal drug user and often disappeared from the village.