Arbovirus surveillance in Laos
 Project Coordinator:
Project Coordinator:
• From October 2021 to May 2022: Dr. Somphavanh Somlor
• From June to October 2022: Dr. Cécile Troupin
Staff members:
Dr. Thonglakhone Xaybounsou
Longthor Vachouaxiong
Kedkeo Intavong
Funding: Supported by the Agence Française du Développement (AFD) through the Ecomore2 project and by the Defense Threat Reduction Agency (DTRA) through the Arboshield Plus project.
Background
Since 2012, the A&EVD laboratory has been providing the Lao Ministry of Health (MOH) with information on circulation of arboviruses within Laos, particularly on Dengue and Chikungunya. For this purpose, the A&EVD laboratory has established a surveillance network for arboviruses in Vientiane capital. In 2015, at the request of the MOH, surveillance was extended to the 2 southern provinces of Laos (Salavan and Attapeu).
Since 2018, it has been extended to 7 more provinces (Champasak, Savannakhet, Luang- Prabang, Xiengkhuang, Phongsaly, Oudomxay and Vientiane province) as a result of the Arboshield project implemented together with the Lao Army Health Service. Thus, the arbovirus surveillance network of IPL currently includes 23 hospitals.
Dengue surveillance
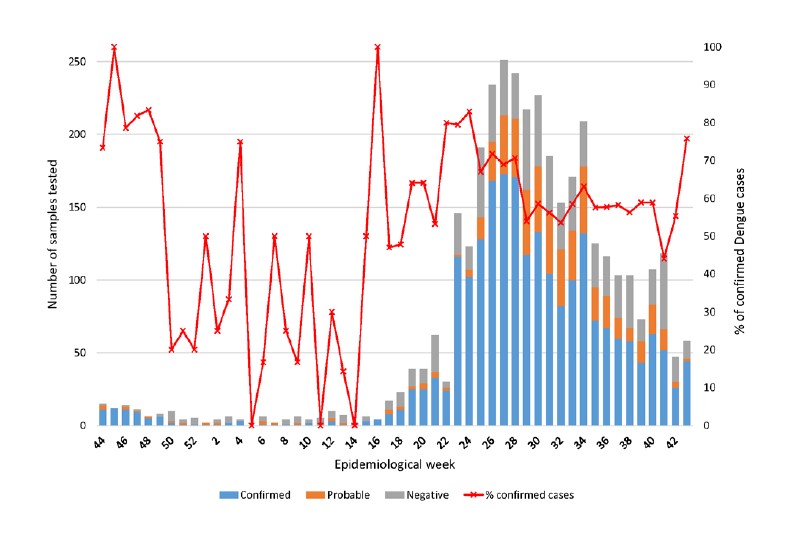
In 2021, 1 683 Dengue fever cases were reported nationwide, including 1 fatal case. In 2022, Laos was facing an important wave of Dengue fever with 28 411 Dengue cases reported until week 41 with 21 fatal cases. From November 2021 to October 2022, 3 566 suspected cases have been investigated at IPL. 76.2% were from our hospitals network in Vientiane Capital (Figure 1). Among them, 62.14% were confirmed as Dengue fever (= RT-PCR or NS1 positive), 15.06 % were considered as probable cases (= presence of Dengue immunological markers (IgM and/or IgG positive)) and 22.80% were classified as suspected cases (= negative for RT-PCR and NS1 and negative for immunological markers (IgG and IgM)) (Figure 2).
Figure 1: Origin of arbovirus surveillance samples received at IPL from November 2021 to October 2022.
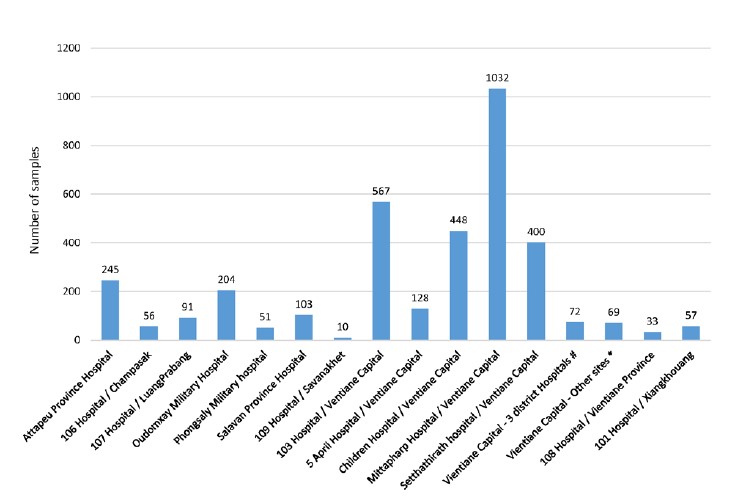
#: 3 district hospitals in Vientiane capital: Sisattanak district hospital (n=38); Hadxayfong district hospital (n=28); Xaythany district hospital (n=6). *: Other sites in Vientiane capital: French Medical Centre (n=37); Institute of Diseases Prevention of Lao Army (n=23); IPL (n=5); Lao-Asean hospital (n=3), Mahosot hospital (n=1).
Figure 2: Number of samples tested at IPL per week from November 2021 to October 2022.
Between November 2021 and October 2022, Dengue serotyping has been performed on 1 160 RT-PCR Dengue positive samples (68.1 % of the samples). Given the significant number of RT-PCR positive cases from Vientiane capital (n= 1 318) only 58.5% of them were serotyped in contrast to all RT-PCR positive cases coming from the other provinces. At the national level, as in 2021, DENV-1 is the predominant serotype circulating in the country and represents 76.5% of RT-PCR positive cases with the DENV-2 serotype representing 23.4%. 2 cases of co-infection by DENV-1 and DENV-2 were also detected. Interestingly, the proportion of DENV-1 and DENV-2 serotypes varied according to the provinces. Thus, in Vientiane capital, Luang Prabang, Vientiane and Xiangkhouang provinces (Central and North) DENV-1 serotype was predominant; whereas in Attapeu, Champasak, Oudomxay provinces (South) DENV-1 and DENV-2 serotypes seemed to circulate at similar level. Finally, in Salavan province (South), DENV-2 serotype was detected in 94.2% of DENV cases.
All results are shared i) on a daily basis with clinicians; ii) on a weekly basis with NCLE and DCDC; and iii) on a monthly basis with DCDC. NCLE and DCDC consolidate testing data from all laboratories and report aggregated data to the MOH. This active surveillance enables the MOH to track the epidemiologic situation of Dengue in Laos.
Beginning of 2023, the Medical Virology and Rabies group will characterize some of the DENV isolates that were detected this year at the genetic level in order to further investigate the DENV genotype circulation in the country.
Differential diagnosis
The algorithm used to investigate dengue suspected cases at IPL includes a differential diagnostic approach. Indeed, Chikungunya has been identified as having high impact in the Lao general population, especially in the southern provinces of Laos. As the diagnostic capacity is still limited in the country and some doctors are unaware of Chikungunya virus (CHIKV) which has some similar symptoms to Dengue, passive surveillance of this virus is an important key for early detection of its introduction and/or circulation in the country.
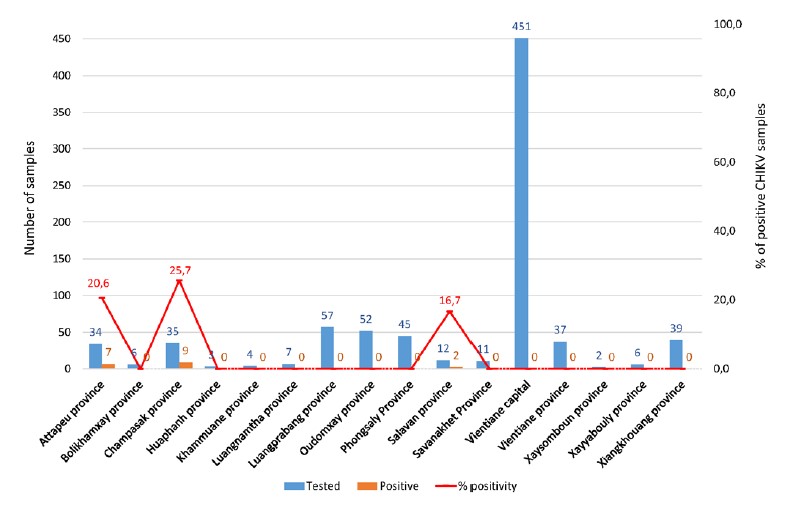
Since June 2022 and the arrival of Dr Cécile Troupin as responsible of the Medical Virology and Rabies group, all samples in which no evidence of DENV infection is detected (negative for RT-PCR and NS1 and negative for immunological markers (IgG and IgM)) and reported as suspected Dengue cases at NCLE have been tested for CHIKV infection by RT-PCR. Samples from all provinces are now investigated, whereas before the samples tested for CHIKV were selected from Southern provinces where the CHIKV cases have been mainly identified. Moreover, a retrospective analysis of Dengue negative cases from November 2021 to May 2022 has been performed. Thus, 821 samples have been tested for the presence of CHIKV and 18 positive cases have been detected from Attapeu (n=7), Champasak (n=9) and Salavan (n=2) provinces (Figure 3).
Figure 3: Number of samples tested and positive for CHIKV infection by province.
Beginning of 2023, the Medical Virology and Rabies group will characterize the CHIKV isolates identified this year at the genetic level in order to investigate the origin of CHIKV circulating in the country.
Evaluation of new diagnosis tools
Zika virus (ZIKV) has been identified as a possible threat in Laos and has already been detected in neighboring countries as Cambodia, Thailand and Vietnam. Moreover, in February 2020, a baby was born with microcephaly in Vientiane. The medical, epidemiological and laboratory investigations demonstrated that this was the first probable congenital Zika syndrome in Laos. This result highlights the need to have an efficient system to test for the presence of ZIKV in samples from suspected cases.
Therefore, a new PCR system developed and routinely used by Institut Pasteur du Cambodge (IPC) allowing the detection of DENV and ZIKV in one PCR has been evaluated by the team at IPL. As no positive ZIKV case has been identified at IPL, it was not possible to evaluate the sensitivity and specificity of this new technique for ZIKV detection, but its limit of detection was evaluated on dilution of ZIKV reference strains and compared to 2 RT-qPCR ZIKV detection systems available in the lab. The limit of detection of this new system is comparable to the 2 others systems previously available in the lab, indicating that it could be used to test samples for the presence of ZIKV in the future.
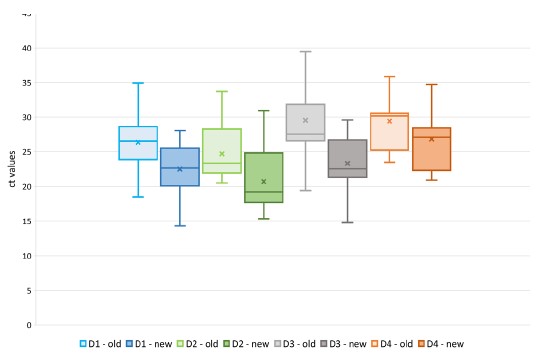
In order to evaluate the DENV detection performance of this new system, a study was performed comparing our current system and the new duplex ZIKV/DENV system on 35 DENV-1 and 24 DENV-2 samples from 2022 and 16 DENV-3 and 16 DENV-4 samples selected from the previous years. For DENV-1 and DENV-2 samples, all of them were detected by both systems. For DENV- 3 samples, 13 were detected by the current system and 16 by the new system. For DENV-4 samples, 15 were detected by the current system and 16 by the new system. According to these results, the new system seems more sensitive, especially on DENV-3 and DENV-4 samples. The ct values obtained from the 2 systems seem also indicate that the new system is more sensitive as its ct values are lower than for the current system (Figure 4).
Figure 4: Ct values obtained on DENV-1, DENV-2, DENV-3 and DENV-4 samples with the old and the new RT-PCR system for DENV.
To confirm this hypothesis, an additional test was performed on samples from week 36 and 37 among which we selected 20 RT-PCR positive samples, 20 RTPCR negative samples with NS1 detection by rapid test and 20 negative samples (by RT-PCR and NS1). These 60 samples were tested by the new DENV RT-PCR system and the positive ones were submitted for serotyping by RT-PCR.
All 20 RT-PCR positive samples were also found positive by the new system. Among the 20 RT-PCR negative/NS1 positive samples, 17 tested positive by the new system and for 11 of them we obtained serotype results. Finally, among the 20 RT-PCR negative samples, 6 tested positive by the new system and for 3 of them we obtained serotype results. In conclusion, these results seem to indicate that the new system is more sensitive than the one currently used by the team.
As a consequence, early in 2023 the new duplex ZIKV/ DENV RT-PCR system will be used to test all Dengue suspected samples and as already started in 2022, all the negative samples will be routinely tested for CHIKV infection.
In parallel, a new PCR system for DENV serotyping has been also evaluated. Indeed, since May 2022, it was impossible to obtain DENV serotype results for DENV RT-PCR positive samples with ct values > 30 which represents around 35% of RT-PCR Dengue positive samples. In order to compare the system used at IPL and a new system developed at IPC, a selection of 20 DENV-1, 20 DENV-2, 10 DENV-3 and 10 DENV-4 positive samples from previous years have been tested in parallel with the old and new systems and all of them were detected by the both system. In addition, all samples submitted for serotyping during week 36 and 37 were tested with this new system (n=73). All of the 50 and 5 samples identified as DENV-1 and DENV- 2, respectively, by the old system were confirmed by the new system. Moreover, interestingly, among the 18 samples for which the serotype was not obtained with the old system (ct>30) the serotype was obtained for 17 with the new system (DENV-1 n=12; DENV-2 n=5) and for only sample the serotype was not obtained. Thus, as this new serotype RT-PCR system seems more efficient than the previous one routinely used in the lab, we started to implement it in the lab in mid-September 2022. Finally, a retrospective analysis of samples for which the DENV serotypes were not obtained with the previous system has been performed (n=159) and we were able to determine the DENV serotype for 93.7 % of them, confirming the better sensitivity of this new system. The 10 samples for which the serotype was not identified are samples with very low viral load (ct > 40).
Finally, in 2023, the team will develop new sequencing tools based on the next-generation sequencing (NGS) to obtain full-length genomes of CHIKV, DENV and ZIKV (if detected).
Acknowledgments
The Medical Virology and Rabies group would like to thanks Dr. Somphavanh Somlor, Phaithong Bounmany, Sitsana Keosenhom, Souksakhone Viengphouthong and Phonesavanh Luangamath for their help with the Arbovirus surveillance activities.







