Ticks and tick-borne diseases in Laos
Principal Investigators: Dr. Ian Satherland (NMRC-A) and Dr. Paul Brey
Project leader: Dr. Kahmsing Vongphayloth
Member of staff: Lae Thutkhin
Background
Ticks are important hematophagous ectoparasites, which play an important role as pests and vectors of a wide range of viral, bacterial and protozoan diseases of humans, livestock, pets, and wild animals. Ticks not only play a role as disease vectors, but also cause direct impact on their hosts, such as blood loss and tick-induced paralysis (Pfaffle et al. 2009, Patil et al. 2012) and cause significant economic losses as a constraint to animal production (Jongejan and Uilenberg 2004).
In Southeast Asia, taxonomically accurate knowledge of tick species and the risk distribution of tick-borne diseases are very poorly characterized. About 104 species of ticks from 12 genera have been known in Southeast Asia, and the most species-rich genus is Haemaphysalis, with about 52 species or 30% of the world fauna (Petney et al. 2007). The relationship between ticks and tick-borne pathogens in this region is largely unknown, even though the presence of these pathogens has been recognized for many years and the number of new pathogens discovered in ticks has increased markedly (Yu et al. 2011).
In Lao PDR, vector borne-diseases are often misdiagnosed and underestimated because of inadequate surveillance networks and laboratory capacity. Up until the present, tick identification, distribution, and their vector status remained unknown. The Nam Theun 2 watershed includes the Nakai Nam Theun National Protected Area (NNT NPA), known as Watershed Management and Protection Authority area .
(WMPA area) located in Nakai district, Khammoune province, covering a total area of 423 000 hectares. This area is considered as a biodiversity hotspot in SE Asia About 6,900 people live in NNT clustered in 31 villages; a density of about 1.95 persons/km2. Villagers in the area mainly rely on a number of forestry-agriculture practices including hunting and gathering of forest and stream products. Water buffalo, cattle, pigs, chickens and dogs are important domestic animals for their livelihood and these valuable animals are often maintained close to human dwelling (http://www.nt2wmpa.gov.la/en/people-and-nature/). Such practices increase the risk of tick-borne disease transmission and other vector borne diseases. Furthermore, several forest workers and scientists working in this zone have been diagnosed and treated for rickettsial disease. Mites and ticks are believed to be the vectors of these diseases and potentially other new emerging infectious diseases such as arboviral diseases. In order to assess tick-borne diseases in Lao PDR, an in-depth assessment of tick species and their pathogens was carried out.
Objectives
+ To describe species composition and distribution of the ticks in the research area.
+ To describe the ticks and their vector status (putative vectors for viruses, rickettsia and bacteria).
+ To develop vector map for ticks and tick-borne diseases in order to provide information on the diseases surveillance and control.
+ To develop local capacity building and competencies on ticks systematics/bionomics and tick-borne disease identification.
Methodology
Building of local capacity and competencies
Training/local capacity building
As previously reported, to improve local capacity and competencies, the first training was held in Vientiane, Lao PDR at IPL from 24th to 28th February 2014, under the title “Intensive Training Course/Workshop on the Taxonomy and Identification of Ticks, Mosquitoes and Sand flies from Lao PDR”.
The course was instructed by Dr. Pollie L.M. Rueda, Ph.D., Research Entomologist, WRBU, Smithsonian Institute (), Dr. Khamsing Vongphayloth, M.D., Research Entomologist, IPL (), Dr. Ian Sutherland, Ph.D., MSPH, Chief of Entomological Sciences, NMRC-A () and Dr. Paul Brey, Ph.D., Research Entomologist, the director of the Institut Pasteur du Laos ().
Sixteen participants from Department of Disease Control (Ministry of Health); CMPE; 103 Military Hospital; Military Institution for Disease Prevention and Control; and 9 provincial divisions of CMPE attended the course (Fig. 1). To our knowledge, this was the first-ever, intensive course dedicated to the taxonomic identification and vector biology of medically important arthropods to be conducted in Lao PDR.
Civilian and military public health professionals from across Lao PDR participated in the joint NMRC-A, IPL, and WRBU Medical Entomology Workshop.
Figure 1: Pictures from February 2014 course.
Institut Pasteur du Laos: Medically important arthropod collection room
In order to preserve specimens for future research and training purposes, a room of medically important arthropod collection has been established with 2 Cornell Cabinets; with 20 drawers each for dry insect specimens such as mosquito adults and one cabinet for specimens preserved in ethanol and slide mounted specimens such as ticks, sand-flies, and mosquito larvae (Fig. 2).
Medically important arthropod collection room in the Institut Pasteur du Laos, with 2 Cornell Cabinets (brown) each with 20 drawers for dry insect specimens, and a cabinet (white) for specimens preserved in ethanol and mounted slides
Figure 2: Medically important arthropod collection room of the Institut Pasteur du Laos.
Tick collection sites
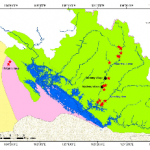
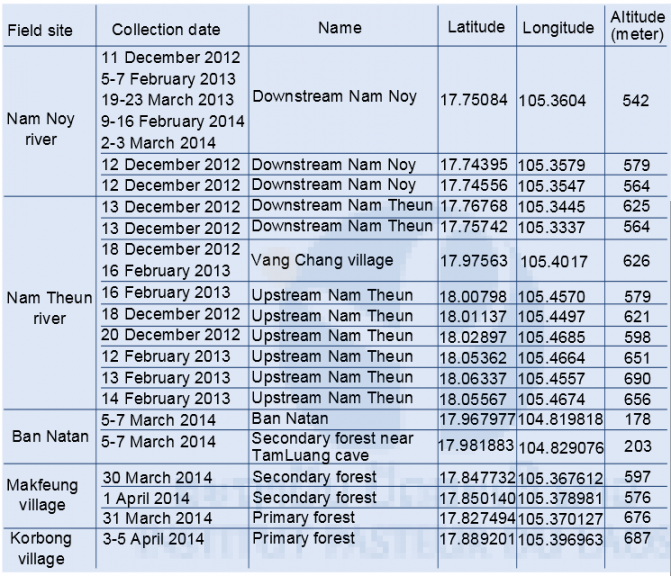
In order to broaden our scope for tick collection and define hot spot areas in Nakai district, Khammoune province, we sampled as many sites as possible, according to the allotted time and logistic difficulties. We investigated the upstream and downstream forest areas of the Nam Theun, Nam Noy rivers, Markfueng and Korbong villages inside WMPA area, and Natan Village in Phou Hin Poun National Protected Area (Fig. 3).
The NNT NPA is under management by the WMPA authority. It is home to wide range of wildlife species including reptiles and amphibians (20 amphibian species, and 31 reptile species), more than 400 species of birds, 92 species of mammals (elephants, muntjac, deer, pangolins, etc.) (http://www.nt2wmpa.gov.la/ en/ecology/). Natan village is located in the Phou Hin Poun National Protected Area (Phou Hin Poun NPA) in Khammouane Province and enclosed by karstic limestone formations and forested mountains. Phou Hin Poun NPA is one of only two NPAs in Lao PDR covering representative samples of the Central Indochina Limestone.
Tick collection was conducted during the dry season; from December to April. The first sampling was started in December 2012 and the last was in April 2014, see Table 1 for the detail of our tick collection sites. During the course of collection in March 2014, temperature for the Nam Noy field site (forest) ranged from 18.5-39°C with a relative humidity (RH) range of 49.5-89.5%. In April 2014, Makfeug village temperature ranged from 19.5-41.5°C with a RH range of 37-80%, while the Korbong village field-site temperature ranged 21-33.5°C with a RH range of 51.5-88%. This is within the normal season averages for this region in the same month [25.5°C; range (20.5-30.5°C), 67%; range (51-83%)] ( Lao PDR climate data, 2011).
- Figure 3: Map of field collection sites in Khammoune Province, Lao PDR.
Tick sampling procedure
Dragging method was used for tick sampling from forest floor and vegetation. White heavy cotton sheets were cut into many sizes: 50 cm, 70 cm, and 100 cm widths X 100 cm long. These makeshift dragnets were swept along the forest ground at around 1-2 m long intervals around natural animal trailways before being examined for ticks. Ticks were removed from the sheets using forceps, transferred to 1.5 ml labeled cryo–tubes, and then transported to the Nakai field Laboratory (Ticks were also collected from some domestic animals by direct hand removal with forceps during our surveys).
In the Lab, ticks were killed by putting in the freezer -20°C for 10 minutes. Then adults, nymphs, and larvae of tick were separated, counted, and subsequently stored at -80°C for further analysis (species identification, pathogen detection, and discovery). All study sites were data-logged for full GPS parameters (see Table 1).
Tick identification
Ticks were identified and grouped under microscope in cooling conditions (on ice pack) by using reference determinations from Dr. Richard G. Robbins of the US Armed Forces Pest Management Board (AFPMB) and together with related keys from Japan, Korea and the Ryukyu Islands (Yamaguti et al. 1972), L. E. Robinson keys for genus Amblyomma (Nuttall et al.) and Thailand (Tanskull and Inlao 1989) for adults of Haemaphysalis ticks. As there are no morphological identification keys available for pre-imago forms of Haemaphysalis spp., larval and nymph stages in this genus were grouped by using their main morphological characteristics, especially the capitulum.
Laboratory procedure for pathogen screening
Sample preparation and RNA/DNA extraction
Tick samples were pooled in to groups of one to ten by species or genus, sex, stage of development, collection period, and site. Specimens were placed in a 1.5 ml vial containing 1 ml of 1X cold Phosphate Buffered Saline (PBS) and Lysing Matrix A zirconium beads (MP Biomedicals). Tick pools were homogenized for 10 min at a vibration frequency of 25/s in a TissueLyser II system (Qiagen). After grinding, beads and tissues were spun down by centrifugation 5 min at 3000 rpm. Total nucleic acid (both DNA and RNA) for bacterial and viral detection by polymerase chain reaction (PCR), 100 μl of each pool were extracted and purified by using NucleoSpin® 8 Virus extraction kit following manufacturer’s protocol. The remaining 400 μl of each pool were stored at –80°C for pathogen isolation.
Reverse transcriptase polymerase chain reaction for viral screening
Multiple sets of primers have been selected and tested for screening arthropods specimens for the presence of flavivirus and alphavirus sequences by means of conventional nested RT-PCR as previously described (Sanchez-Seco et al. 2001 and 2005). In order to improve the sensitivity of the flavivirus screening, a real time RT-PCR system is under evaluation (Moureau 2007). As planned in the project, positive samples for any of these two arbovirus genera detected through an initial screening were re-analyzed by specific real time RT-PCR to attempt to identify the viral species. Such methods are already available for detection of the main arboviruses of human health concern in SE Asia: dengue (all serotypes), Japanese encephalitis, and chikungunya virus.
Virus isolation
Positive samples for any viral markers were inoculated onto both mosquito (Aedes albopictus derived C6/36) and mammalian (African green monkey VERO E6) cell lines. Briefly, sub-confluent cells monolayers prepared in 25 cm2 culture flasks were washed once with 3 ml of 1X DPBS. After removing the washing solution, cells were inoculated with 100 µl of each positive pool supernatant diluted with 1 ml of cold culture medium filtered through a 0.22 µm Millipore filter unit. After two hours of contact, the inocula were removed and replaced by 5 ml of culture media completed by fetal calf serum. Cultures were maintained for 5 days and checked daily under a microscope for presence of cytopathic effect (CPE). Up to four blind passages were performed to follow up of culture negative for CPE. Cells and supernantant were preserved frozen at -80°C for a retrospective control of the culture by pan-flavi or pan-alpha RT-PCR.
Bacterial screening
To investigate the occurrence of Spotted Fever Group (SFG) Rickettsia in ticks in Laos a molecular screening approach targeting the 17kDa gene was taken (Jiang et al. 2004). In order to identify novel as well as known human pathogenic species a previously established and validated quantitative real-time PCR (qPCR) assay was adapted for the use with DNA from tick pools (Jiang et al. 2004, Mayxay et al. 2013). After severe inhibition of the qPCR reaction was identified in preliminary experiments the final protocol was adapted for the use with diluted (1:10) tick-DNA and the inclusion of bovine serum albumin (BSA) to minimizing the effects of inhibitors.
Further, a subset of pools was investigated for the presence of Anaplasma phagocytophilum (136/768, 17/7%; Joshua et al. 2004), Ehrlichia chaffeensis (85/768, 11%; Amanda et al. 2003) and Coxiella burnetii (68/768; 8.8%; Fournier et. al. 2010), all significant human pathogens in many parts of the world (Pritt et al. 2011, Dumler et al. 2005).
The validity of results was ensured by the inclusion of positive and negative results into each run. The possibility for cross-contamination was limited due tostrict physical separation of DNA preparation, pre-and post PCR areas as well as the use of uracil-DNA glycosylase.
Results
Pending
Acknowledgements
This study was supported by a grant form the United States Department of Defense Global Emerging Infections System (GEIS)
+ We also thank Dr. Richand Robbins of the United States Department of Defense Pest Management Board and Dr. Pollie Rueda, Walter Reed Biosystematies Unit for their collaboretion and assistance
References
Courtney JW, Kostelnik LM, Zeidner NS, Massung RF. (2004). Multiplex real-time PCR for detection of anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol 42:3164-3168.
Dittrich S, Phommasone K, Anantatat T, Panyanivong P, Slesak G, Blacksell SD, Dubot-Peres A, Castonguay-Vanier J, Stenos J, Newton PN, Paris DH. (2014). Rickettsia felis Infections and Comorbid Conditions, Laos, 2003-2011. Emerg Infect Dis 20:1402-1404.
Dittrich S, Rattanavong S, Lee SJ, Panyanivong P, Craig SB, Tulsiani SM, Blacksell SD, Dance DAB, Dubot-Pérès A, Sengduangphachanh A, Phoumin P, Paris DH, Newton PN. (submitted). Rickettsia and leptospira as neglected but treatable causes of central nervous system infections. Lancet Global Health.
Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS. (2005). Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis 11:1828-1834.
ournier PE, Thuny F, Richet H, Lepidi H, Casalta JP, Arzouni JP, Maurin M, Celard M, Mainardi JL, Caus T, Collart F, Habib G, Raoult D. (2010). Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis 51:131-140
Hildebrandt A, Kramer A, Sachse S, Straube E. (2010). Detection of Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus ticks in a region of Middle Germany (Thuringia). Ticks Tick Borne Dis 1:52-56.
Jiang J, Chan TC, Temenak JJ, Dasch GA, Ching WM, Richards AL. (2004). Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am J Trop Med Hyg 70:351-356.
Jongejan, F. and G. Uilenberg (2004). “The global importance of ticks.” Parasitology 129 Suppl: S3-14.
Kumsa B, Socolovschi C, Raoult D, Parola P. (2014). Spotted fever group rickettsiae in ixodid ticks in Oromia, Ethiopia. Ticks Tick Borne Dis.
Loftis AD, Massung RF, Levin ML. (2003). Quantitative real-time PCR assay for detection of Ehrlichia chaffeensis. J Clin Microbiol 41:3870-3872.
Mayxay M, Castonguay-Vanier J, Chansamouth V, Dubot-Pérés A, Paris DH, Phetsouvanh R, Tangkhabuanbutra J, Douangdala P, Inthalath S, Souvannasing P, Slesak Gn, Tongyoo N, Chanthongthip A, Panyanouvong P, Sibounheuang B, Phommasone K, Dohnt M, Phonekeo D, Hongvanthong B, Xayadeth S, Ketmayoon P, Blacksell SD, Moore CE, Craig SB, Burns M-A, von Sonnenburg F, Corwin A, de Lamballerie X, Gonz•lez IJ, Christophel EM, Cawthorne A, Bell D, Newton PN. (2013). Causes of non-malarial fever in Laos: a prospective study. The Lancet Global Health 1:e46-e54.
Nuttall, G. H. F., W. F. Cooper, C. Warburton, L. E. Robinson and D. R. Arthur Ticks: pt.IV. The genus Amblyomma, 1926, Cambridge University Press.
Park, S. W., B. G. Song, E. H. Shin, S. M. Yun, M. G. Han, M. Y. Park, C. Park and J. Ryou (2014). “Prevalence of severe fever with thrombocytopenia syndrome virus in Haemaphysalis longicornis ticks in South Korea.” Ticks Tick Borne Dis 5(6): 975-977.
Parola P. (2011). Rickettsia felis: from a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin Microbiol Infect 17:996-1000.
Parola P, Cornet JP, Sanogo YO, Miller RS, Thien HV, Gonzalez JP, Raoult D, Telford IS, Wongsrichanalai C. (2003). Detection of Ehrlichia spp., Anaplasma spp., Rickettsia spp., and other eubacteria in ticks from the Thai-Myanmar border and Vietnam. J Clin Microbiol 41:1600-1608.
Patil, M. M., B. N. Walikar, S. S. Kalyanshettar and S. V. Patil (2012). “Tick induced facial palsy.” Indian Pediatr 49 (1): 57-58. Petney, T. N., G. V. Kolonin and R. G. Robbins (2007). “Southeast Asian ticks (Acari: Ixodida): a historical perspective.” Parasitol Res 101 Suppl 2: S201-205.
Pfaffle, M., T. Petney, M. Elgas, J. Skuballa and H. Taraschewski (2009). “Tick-induced blood loss leads to regenerative anaemia in the European hedgehog ( Erinaceus europaeus).” Parasitology 136(4): 443-452.
Phongmany S, Rolain JM, Phetsouvanh R, Blacksell SD, Soukkhaseum V, Rasachack B, Phiasakha K, Soukkhaseum S, Frichithavong K, Chu V, Keolouangkhot V, Martinez-Aussel B, Chang K, Darasavath C, Rattanavong O, Sisouphone S, Mayxay M, Vidamaly S, Parola P, Thammavong C, Heuangvongsy M, Syhavong B, Raoult D, White NJ, Newton PN (2006). “Rickettsial infections and fever, Vientiane, Laos.” Emerg Infect Dis. Feb;12(2):256-62.
Pritt BS, Sloan LM, Johnson DK, Munderloh UG, Paskewitz SM, McElroy KM, McFadden JD, Binnicker MJ, Neitzel DF, Liu G, Nicholson WL, Nelson CM, Franson JJ, Martin SA, Cunningham SA, Steward CR, Bogumill K, Bjorgaard ME, Davis JP, McQuiston JH, Warshauer DM, Wilhelm MP, Patel R, Trivedi VA, Eremeeva ME. (2011). “Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, (2009).” N Engl J Med 365:422-429.
Sanchez-Seco, M. P., D. Rosario, C. Domingo, L. Hernandez, K. Valdes, M. G. Guzman and A. Tenorio (2005). “Generic RT-nested-PCR for detection of flaviviruses using degenerated primers and internal control followed by sequencing for specific identification.” J Virol Methods 126(1-2): 101-109.
Sanchez-Seco, M. P., D. Rosario, E. Quiroz, G. Guzman and A. Tenorio (2001). “A generic nested-RT-PCR followed by sequencing for detection and identification of members of the alphavirus genus.” J Virol Methods 95 (1-2): 153-161.
Tanskull, P. and I. Inlao (1989). “Keys to the adult ticks of Haemaphysalis Koch, 1844, in Thailand with notes on changes in taxonomy (Acari: Ixodoidea: Ixodidae).” J Med Entomol 26(6): 573-601.
Yamaguti, N., V. J. Tipton, H. L. Keegan, S. Toshioka and B. Y. U. P. UT. (1972). Ticks of Japan, Korea, and the Ryukyu Islands. Volume 15, Number 1, Defense Technical Information Center.
Yun, S. M., W. G. Lee, J. Ryou, S. C. Yang, S. W. Park, J. Y. Roh, Y. J. Lee, C. Park and M. G. Han (2014). “Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013.” Emerg Infect Dis 20(8): 1358-1361.
Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. (2011) Fever with thrombocytopenia associated with a novel bunyavirus in China. NEJ Med. 364:1523-1532.