Antimalarial Drug Therapeutic Efficacy Study
 Project Coordinator: Dr. Moritoshi IWAGAMI
Project Coordinator: Dr. Moritoshi IWAGAMI
Partners: Dr. Center of Malariology, Parasitology and Entomology (CMPE), Ministry of Health, Vientiane, Lao PDR
2019-2022
Savannakhet Provincial Health Department
Salavan Provincial Health Department
Champasak Provincial Health Department
2021-2022
Savannakhet Provincial Health Department
Sekong Provincial Health Department
Attapeu Provincial Health Department
Staff members in Parasitology lab:
Dr. Phonepadith Khattignavong, Dr. Sengdeuane Keomalaphet, Dr. Phoyphaylinh Prasayasith, Ms. Pheovaly Soundala, and Ms. Sonesimmaly Sannikone
Finance and administration:
Dr. Antoine des Graviers and Ms. Phonesavanh Vilayseng
Background
Malaria, caused by the protozoan parasite genus Plasmodium, has long been one of the most medically important parasitic diseases in the Greater Mekong Sub-region (GMS) including Lao PDR. Nevertheless, morbidity and mortality of malaria have significantly decreased in the GMS over the last decade due to extensive efforts by the governments, partners and international organizations, such as World Health Organization, Global Fund, Bill & Melinda Gates Foundation [1]. The Lao Ministry of Health adopted an ambitious goal to eliminate Plasmodium falciparum malaria by 2025 and all forms of human malaria by 2030. Artemisinin-based combination therapy (ACT) has been used for malaria treatment worldwide. In Lao PDR, Coartem (artemether-lumefantrine), one of the ACTs, has been used for the treatment of P. falciparum malaria since 2004. However, clinical artemisinin resistance (or delayed parasite clearance) in P. falciparum was first reported in Pailin, the western part of Cambodia in 2009 [2] and gradually spread or emerged in the neighboring countries, which is a serious threat for malaria control and elimination in the GMS and globally.
Percentages of the k13 mutations detected were 55.7% (660/1,185) in 2015, 44.6% (179/401) in 2016 and 23.9% (109/457) in 2017. The predominant mutation was C580Y, which was also predominant in Cambodia, followed by Y493H, R539T and P574L. The percentage of the mutations was higher in the two southernmost provinces, Champasak and Attapeu. In Phongsaly, all the P. falciparum samples (3/3) possessed the C580Y in 2017. This study suggested the percentages of the k13 mutations seemed to be decreasing in Lao PDR. However, caution is needed because this tendency might be due to the relatively large number of P. falciparum samples from Savannakhet province with lower percentages of the mutation: 21.1% (35/166) in 2016 and 5.3% (14/266) in 2017. In addition, the mutation rate as high as 77.0% (47/61) was still observed in the southernmost province, Champasak in 2017. On the other hand, an antimalarial drug therapeutic efficacy study (TES) conducted by CMPE demonstrated that 10%-14% of cases were artemisinin-resistant in 2013-2017 [1].
This TES result, together with the prevalence of the k13 mutations suggested that the efficacy of Coartem is slightly decreasing and the partner drug, lumefantrine is the main actor to clear malaria parasites in the patients. However, no molecular marker has been identified for lumefantrine so far.
Objective
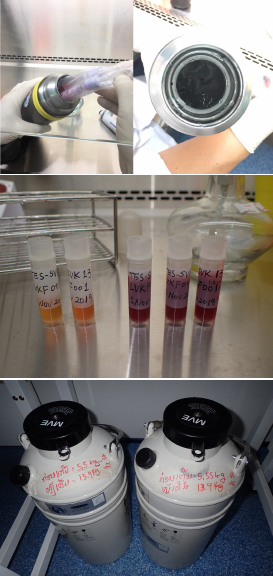
The objective of this study is to collect blood samples (living Plasmodium falciparum) from malaria patients in the field for laboratory analysis to identify and map resistant markers in the artemisinin partner drug, lumefantrine that is currently used in Lao PDR and many African countries for the first-line treatment of malaria. The main role of the Lao-Japan Parasitology Lab, IPL in this study is to preserve the sample of P. falciparum in liquid nitrogen in collaboration with CMPE and the local healthcare facilities in the study sites. The laboratory analysis will be conducted at Institut Pasteur du Cambodia (IPC).
Methodology
The study sites of this project are at public hospitals (provincial hospitals and district hospitals) in malaria-endemic areas in Savannakhet, Salavan and Champasak provinces in 2019-2020 and Savannakhet, Sekong and Attapeu provinces in 2021-2022. Live P. falciparum samples are collected from malaria patients who participated in this study. The collected samples (approximately 2mL of fresh blood sample in EDTA tube) are sent by public bus in a Thermos bottle with ice water to IPL and red blood cells are preserved in liquid nitrogen with a freezing solution. The live P. falciparum samples will be sent to IPC with dry shippers (special liquid nitrogen tanks) at the end of the project by air or land. Ethical clearance for this study was obtained by CMPE.
Results and future plan In 2019, a total of 50 samples (47 samples: Savannakhet; 3 samples: Salavan) were collected in this study and the red blood cells were stored in the liquid nitrogen tanks. In 2020, 29 samples (26 samples: Savannakhet; 3 samples: Salavan) were collected. In 2021, 8 samples were collected in Attapeu. Serum samples and the remaining red blood cells were preserved in a freezer (-30oC) and filter paper, respectively. Currently, it is impossible to send the frozen live P. falciparum samples to IPC because no airline or courier company that accepts frozen samples is available between Lao PDR and Cambodia due to the COVID-19 pandemic. The land border is also currently closed.
Results and future plan
In 2019, a total of 50 samples (47 samples: Savannakhet; 3 samples: Salavan) was collected in this study and the red blood cells were stored in the liquid nitrogen tanks. In 2020, 29 samples (26 samples: Savannakhet; 3 samples: Salavan) were collected. In 2021, 8 samples were collected in Attapeu. Serum samples and the remaining red blood cells were preserved in a freezer (-30oC) and filter paper, respectively. Currently, it is impossible to send the frozen live P. falciparum samples to IPC because no airline or courier company that accepts frozen samples is available between Lao PDR and Cambodia due to the COVID-19 pandemic. The land border is also currently closed.
Financial support
This study is financially supported by World Health Organization
References
1. World Health Organization, World Malaria Report 2019, Geneva: World Health Organization, 2019.
2. Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. New England Journal of Medicine, 361(5): 455-467, 2009.
3. Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, et al., A molecular marker of artemisinin-resistant Plasmodium falciparum malaria, Nature, 505(7481): 50-55, 2014.
4. Ménard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, et al., A Worldwide Map of Plasmodium falciparum K13-Propeller Polymorphisms. New England Journal of Medicine, 374(25): 2453-2464, 2016.
5. Iwagami M, Nakatsu M, Khattignavong P, Soundala P, Keomalaphet S, Lorpachan L, Xangsayalath P, Matsumoto‑Takahashi E, Pommelet V, Hongvanthong B, Brey PT, Kano S. Heterogeneous distribution of k13 mutations in Plasmodium falciparum in Laos. Malaria Journal, 17: 483, 2018.







