SARS-CoV-2 LAMP Study
 Project coordinator: Dr. Moritoshi Iwagami
Project coordinator: Dr. Moritoshi Iwagami
Staff members in Parasitology Lab: Dr. Phonepadith Khattignavong, Dr. Sengdeuane Keomalaphet, Dr. Phoyphaylinh Prasayasith, Ms. Pheovaly Soundala, Ms. Sonesimmaly Sannikone
Staff members in Arbovirus & Emerging viral diseases laboratory Lab: Dr. Vincent Lacoste Dr. Somphavanh Somlor
Background
This is a collaborative study between the Parasitology Lab and Arbovirus & Emerging viral diseases laboratory Lab. Loop-mediated isothermal amplification (LAMP) is one of the nucleic acid amplification methods similar to PCR. We conducted a performance evaluation of a Reverse-transcription LAMP (RT-LAMP) kit for SARSCoV- 2 (LoopampTM SARS-CoV-2 Detection Kit, Eiken Chemical, Co., Ltd., Japan) by comparing it with the performance of RT-PCR for SARS-CoV-2.
Methodology
A total of 302 clinical samples were used for this study. 237 samples were collected by IPL while 65 samples were collected by National Center for Laboratory and Epidemiology (NCLE) for the SARS-CoV-2 test. RNA was extracted from 150μL of the swab samples using NucleoSpin® RNA Virus (MACHEREY-NAGEL, Germany). The RT-qPCR was performed by the Berlin method [1] while the RT-LAMP was performed according to the instructions of the RT-LAMP kit. Briefly, 15 μL primer mix and 10μL RNA template were added in the reaction tube with the kit and mixed well. Dried enzymes are applied inside of a cap of the reaction tubes. The reaction tube was incubated at 62.5oC for 35 min and then inactivated at 95oC for 2 min by using a Real-Time Turbidimeter LA-500 (Eiken Chemical Co. Ltd, Japan) (Fig. 4). Sensitivity, specificity, and positive and negative predictive values were calculated using the data of RTPCR as a reference.
Results and discussion
 The sensitivity and specificity of the RT-LAMP test were 93.2% and 99.1%, respectively (Table 1). Positive and negative predictive values of the RT-LAMP kit were 97.6% and 97.2%, respectively. The sensitivity of the RT-LAMP kit was slightly lower than that of the RT-PCR. However, we found that the RT-LAMP kit is simple and quick to perform. In addition, all the reagents including an RNA extraction kit can be stored in the refrigerator (2- 8oC). Thus, this RT-LAMP kit can be used in resource-limited settings such as small clinics and quarantine centers.
The sensitivity and specificity of the RT-LAMP test were 93.2% and 99.1%, respectively (Table 1). Positive and negative predictive values of the RT-LAMP kit were 97.6% and 97.2%, respectively. The sensitivity of the RT-LAMP kit was slightly lower than that of the RT-PCR. However, we found that the RT-LAMP kit is simple and quick to perform. In addition, all the reagents including an RNA extraction kit can be stored in the refrigerator (2- 8oC). Thus, this RT-LAMP kit can be used in resource-limited settings such as small clinics and quarantine centers.
Financial Support:
Japan International Cooperation Agency (JICA)
Reference
1. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al, Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill, 25(3): 2000045, 2020.
Scientific communications
Oral presentation:
1. Moritoshi Iwagami, Masami Nakatsu, Sengdeuane Keomalaphet, Phonepadith Khattignavong, Pheovaly Soundala, Phoyphaylinh Prasayasith, Bouasy Hongvanthong, Viengxay Vanisaveth, Paul T. Brey, Shigeyuki Kano, Molecular surveillance of the distribution of artemisinin-resistant Pf parasites in Laos during 2015- 2017, The 90th Annual Meeting of the Japanese Society of Parasitology and the 32nd Annual Meeting of the Japanese Society of Clinical Parasitology, Nara, Japan, 15-17, April 2021 (Online meeting)
2. Current situation and challenges of fieldwork in Lao PDR under the COVID-19 pandemic: A report from Laos, Moritoshi Iwagami, The 80th East Branch Meeting of The Japanese Society of Parasitology, Dokkyo Medical University, Tochigi, Japan, 30th, October 2021 (Online meeting)
Poster presentation:
1. Moritoshi Iwagamim, Masami Nakatsu, Phonepadith Khattignavong, Sengdeuane Keomalaphet, Pheovaly Soundala, Kanako Komaki-Yasuda1, Paul T. Brey, Shigeyuki Kano, Performance evaluation of malaria- LAMP tests using dried-blood samples of malaria patients in Laos in 2015, The 62nd Annual Meeting for the Japanese Society of Tropical Medicine, Tohoku University, Miyagi, Japan, 3-5, November 2021 (Online meeting)
2. Moritoshi Iwagami, Masami Nakatsu, Phonepadith Khattignavong, Sengdeuane Keomalaphet, Pheovaly Soundala, Kanako Komaki-Yasuda, Paul T. Brey, Shigeyuki Kano, Sensitivity and specificity of LAMP tests for malaria diagnosis using dried-blood samples of suspected malaria patients in Lao PDR, Joint International Tropical Medicine Meeting 2021 (JITMM Virtual 2021), Bangkok, Thailand, 15-17 December 2021 (Online meeting)
Publication
Moritoshi Iwagami, Current Situation and Challenges of Parasitic Diseases in Lao PDR- Schistosomiasis, Opisthorchiasis, and others (in Japanese), Modern Media, 66(12): 375-388, 2020 (https://www.eiken.co.jp/ uploads/modern_media/literature/P23-36.pdf)
Table:
Table 1. Summary of performance of the SARS-CoV-2 LAMP Kit
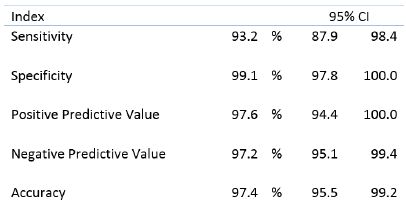
LoopampTM SARS-CoV-2 Detection Kit, Eiken Chemical Co., Ltd., Japan. The performance of the SARS-CoV-2 LAMP Kit was calculated based on the results of the RT-PCR.
Figure Legend:
Figure 1. Scheme of the 5-ALA asymptomatic malaria study
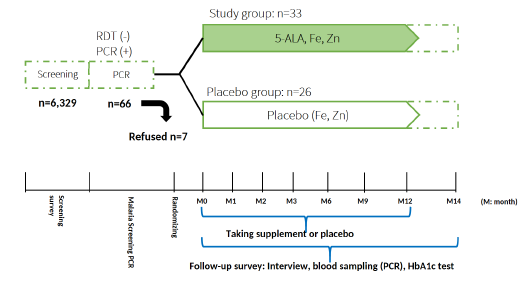
Figure 2. Study sites of the 5-ALA asymptomatic malaria study
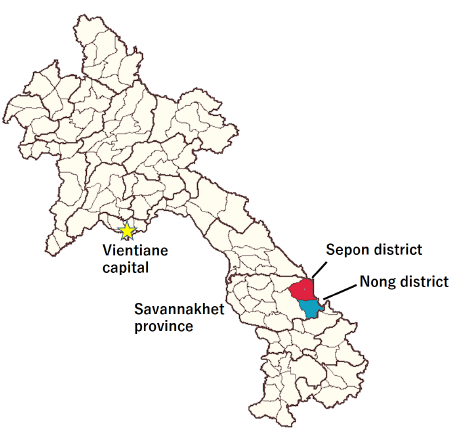
Figure 3. 5-ALA supplement or Placebo

The appearance of 5-ALA supplement and Placebo are
identical to each other.
Figure 4. Device for the LAMP test

Real-time Turbidimeter LA-500, Eiken Chemical Co., Ltd., Japan.
IPL publication 2021
Virachith S, Pommelet V, Calvez E, Khounvisith V, Sayasone S, Kounnavong S, Mayxay M, Xangsayarath P, Temmam S, Eloit M, Escriou N, Rose T, Vongphayloth K, Hübschen JM, Lacoste V, Somlor S, Phonekeo D, Brey PT, Black AP. Low seroprevalence of COVID-19 in Lao PDR, late 2020. Lancet Reg Health West Pac. 2021 Aug;13:100197.
Cheung D, Khounvisith V, Sitbounlang P, Douangprachanh S, Virachith S. Arounlangsy P, Hübschen JM, Paboriboune P, Black AP. Knowledge, attitude and practice towards liver cancer and liver cancer screening among HBV and HCV patients in Vientiane, Lao People’s Democratic Republic: a cross-sectional study. Clinical and Experimental Hepatology. August 2021.
Hefele L, Xaydalasouk K, Kleine D, Homasana A, Xayavong D, Syphan S, Hübschen JM, Muller CP, Black AP. Seroprevalence of measles and rubella antibodies in vaccinated and unvaccinated infants in the Lao People’s Democratic Republic. Int. J. Inf. Dis, July 2021
Snoeck C, Evdokimov K, Xaydalasouk K, Mong Khoune S, Sausy A, Vilivong K, Pauly M, Hübschen JM, Billamay S, Muller C, Black AP. Epidemiology of acute respiratory viral infections in children in Vientiane, Lao People’s Democratic Republic. J. Med. Virology, April 2021.
Xaydalasouk K, Sayasinh K, Hübschen J, Khounvisith V, Keomany S, Muller C, Black AP. Age-stratified seroprevalence of vaccine-preventable infectious disease in Saravan, Southern Lao People’s Democratic Republic. International Journal of Infectious Diseases. April 2021.
Fuchs F, Pauly M, Black AP, Hübschen JM. Seroprevalence of ToRCH pathogens in Southeast Asia. Microorganisms. March 2021.
Pollack E, Kunlaya K, Keokhamphoui C, Souksakhone C, Chanthavilay P, Sayasone S, Black AP, Nouanthong P. Suboptimal knowledge of hepatitis B infection and concerns regarding HBV vaccination among blood donors in Lao People’s Democratic Republic (PDR). Lao Medical Journal, 2021.
Khampanisong P, Pauly M, Nouanthong P, Vickers MA, Virachith S, Xaydalasouk K, Black AP, Muller CP, Hübschen JM. The waning of Maternal Antibodies Against Measles Suggests A Large Window of Susceptibility in Infants in Lao People’s Democratic Republic. Pathogens, 2021.
Calvez E, Vetsaphong P, Somlor S, et al. First probable case of congenital Zika syndrome in Lao People’s Democratic Republic. Int J Infect Dis. 2021;105:595-597. doi:10.1016/j.ijid.2021.03.019
Moritoshi Iwagami, Current Situation and Challenges of Parasitic Diseases in Lao PDR- Schistosomiasis, Opisthorchiasis, and others (in Japanese), Modern Media, 66(12): 375-388, 2020 (https:// www.eiken.co.jp/uploads/modern_media/literature/ P23-36.pdf)
Ong KIC, Khattignavong P, Keomalaphet S, Iwagami M, Brey PT, Kano S, Jimba M. Health-seeking behaviors in a malaria-endemic district in Lao People’s Democratic Republic: a mixed-methods study. BMJ Open 2021







