Kinetic study of cellular viremia of dengue virus in Vientiane Capital, Lao PDR Research program PTR-491
Funded by the International Division, Institut Pasteur, Paris In collaboration with the Arbovirus and vectors unit and Pathogen Genotyping Pole, Institut Pasteur, Paris.

Project coordinator: Marc GRANDADAM, Somphavanh SOMLOR
Member of staff: Thonglakhone Xaybounsou, Sitsana Keosenhom and Phaithong Bounmany
Background
Dengue is considered as the most important arthropod-borne viral disease worldwide due its human morbidity and mortality. In urban and rural settings, dengue transmission cycle can only be maintain between Aedes vectors a humans without the involvement of intermediate amplifying vertebrate (s). Thus, virus maintenance directly depends on the duration of the persistence of the virus in human blood compartments. Dengue epidemiology relies on data regarding the length of free virus viremia (i.e. presence of the virus in serum or plasma) which supposed to last up to 5 or 8 days after symptom onset.
However, dengue virus can be isolated from plasma and peripheral blood mononuclear cells (PBMCs). Currently, there are no precise data on the duration of dengue virus cellular viremia. The role of dengue cellular viremia in the maintenance of dengue transmission cycle has not been determined yet.
The aim of the PTR-491 was to improve knowledges on dengue virus viremias to determine the putative interest to test cellular viremia in dengue suspected cases and evaluate its impact on dengue epidemiology. To fulfill these objectives, different approaches were envisaged:
(i) a retrospective study on dengue suspected cases between 2012 and 2015 to compare plasmatic and cellular dengue viremia
(ii) a prospective longitudinal study on confirmed cases to assess the kinetic of the two types of dengue viremia.
Once the cellular viremia kinetic has been documented, experimental infections of Aedes mosquitoes should be designed to demonstrate the capacity of the infected PBMCs to transmit dengue infection to the actual vectors of the virus.
A total of 452 dengue confirmed cases were selected with respectively 264 and 188 for the retrospective and the prospective studies.
The retrospective study allowed a comparison of different dengue profiles (positive plasmatic viremia; positive NS1 antigenemia; positive plasmatic viremia and positive NS1 antigenemia). PBMCs were often found positive in subgroups displaying a positive plasmatic viremia. Among patients with only NS1 antigenemia, up to 55% were found positive for PBMCs infection. More interestingly, an additional subgroup of patients lacking any direct diagnostic markers (6/124; 4.8%) were found positive for dengue infection in PBMCs. Viral isolation could be achieved from these PBMCs excluding a possible contamination of molecular detection. Altogether, these results highlight the interest of dengue cellular detection in “late patients”, but also suggest that cellular viremia may precede plasmatic viremia and/or NS1 antigenemia.
A total of 188 confirmed cases were followed for this study. These volunteers were sampled every three days until all direct markers of dengue infection became negative (plasmatic viremia; cellular viremia; NS1 antigenemia). The mean duration the plasmatic viremia duration was 4 days (Min 0 – 16 Max). About 70% of the patients displayed a cellular viremia within the 7 days after symptom onset, 4% were still positive in PBMCs from 8 to 14 days and 1% remained positive more than 15 days after the beginning of the acute phase.
The kinetic of dengue virus viremias could only be established for the serotypes 1, 2 and 4 as DENv-3 has not circulated in Vientiane Capital since December 2013. No significant difference could be evidenced between these serotypes.
All these results strongly support the interest of dengue virus cellular viremia to reinforce the efficiency of the direct diagnosis of dengue infection and raise questions on its contribution to virus maintenance at least in urban areas.
All these results strongly support the interest of dengue virus cellular viremia to reinforce the efficiency of the direct diagnosis of dengue infection and raise questions on its contribution to virus maintenance at least in urban areas.

Picture 1: Sorting of Aedes mosquito specimens after experimental blood feeding infection in IPL BSL3
Consolidated Asian Network for Zika virus surveillance in the Lao PDR (CANZ-Lao)
Research program funded by the US DOD
In collaboration with NMRC-A, Singapore.
Vector-borne diseases, including Zika, constitute a significant infectious disease risk for local populations. In Laos, definitive diagnosis, especially for Zika, is often not available for vector-borne illnesses, so the infectious diseases that are a threat the population are not well defined. In order to identify common and emerging vector-borne pathogens in Laos, NMRC-A Singapore (SG) and IP Lao PDR have established a study to assess and survey the distribution and infection status of Zika virus. The project aimed to set up retrospective and prospective investigations of human and mosquito specimen to determine the rate of circulation of Zika virus in Lao PDR.
During this last quarter of the project, prospective human surveillance continued using the new algorithm for dengue suspected cases investigation set up thanks to CANZ-Lao project. Plaque reduction neutralization tests continued to finalize the seroprevalence study. Finally, new entomologic investigations were performed in new locations.
Complementary investigations on human and mosquito specimen were performed during the last quarter of the project in order to document a possible active circulation of Zika virus in Laos. A global analysis of the data gathered during the project has been made to draw conclusion regarding the present risk for Zika virus in the area studied and draw some perspectives.
Zika indirect makers detection in human samples
A specific ethical clearance has been requested and obtained from the National Ethic Committee for human research of the Lao Ministry of health to proceed to the retrospective and prospective investigations on human samples. Different collections of human sera or plasma were re-investigated to establish a first map, even partial, of the possible distribution areas of Zika virus in Laos. During the initial studies, informed consent was obtained for all participants included in these different series before sampling, allowing the use of their anonymized samples for improving diagnosis and general knowledge of arboviral infection
Anti-Zika virus antibodies detection in human samples
Retrospective serologic studies
Antibody specificity of all positive sera were tested against a representative strain of the Asian genotype of Zika virus and in parallel against a Lao dengue virus 1 isolate selected in the IPL virus repository to determine the neutralization threshold. Sera displaying a significant neutralization titer were used as positive control in the different series of PRNT as an external quality control.
A first series of 503 sera from patients (ages ranging from 5 to 18 years) from Vientiane Province presenting a fever-rash syndrome, were tested for the presence of anti-Zika virus IgM and IgG by means of in-house ELISA tests.
Table 1: Antibodies reacting against Zika virus antigen in patients presenting a fever-rash syndrome
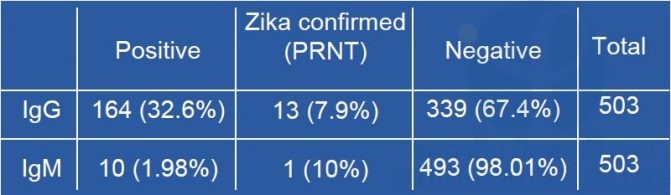
Table 1: Antibodies reacting against Zika virus antigen in patients presenting a fever-rash syndrome
As shown in table 1, antibodies reacting with the Zika virus antigen could be found in 1.9% and 32% of the subjects in IgM and IgG respectively. In this series of samples, all the 164 sera positive for the presence of anti-Zika virus IgG and the 10 sera positive in MAC-ELISA, were submitted to the PRNT tests using Vero E6 cell line exposed to serial dilutions of the sample mixed with a calibrated dose of each virus. Plates were stained after seven days of incubation (Figure n°5). The first series of neutralization performed during a previous quarter of the project allowed to adopted criteria of interpretation established after the Zika outbreak on Yap island [Duffy et al, 2009]. A serum was considered as positive if the last dilution giving at least 50% of reduction of the viral inoculum was above or equal to 20 and if this titer is at least four time the dengue PRNT50. Among these samples, only 7.9% of the IgG positive and 10% of the IgM positive sera could be confirmed by PRNT. The actual Zika virus seroprevalence rates in this group were respectively 2.6% and 0.2% in IgG and IgM.
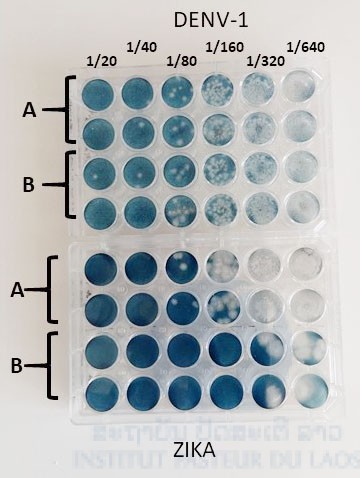
Figure n°5: Zika virus Plaque reduction neutralization assay performed on Vero E6 cell line.
A second series of human samples has been investigated for the presence of anti-Zika virus IgM. A total of 801 dengue suspected cases were randomly selected among the patients that displayed no direct marker (RT-PCR; NS1 antigen; viral culture) of dengue infection. These samples were selected within the samples collected in 2013 and 2016. Among them, only one serum collected in 2013 displayed a significant neutralization titer (Table 2). In this subgroup, the IgM seroprevalence rate was 0.19% after PRNT analysis. None of the sera from 2016 found positive with the MAC-ELISA displayed a significant neutralization titer.
Table 2: PRNT tests of sera from dengue suspected cases displaying anti-Zika virus reactive IgM
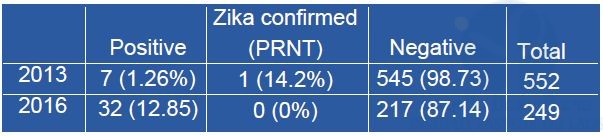
Serological traces of the presence of Zika virus have been found within the two series of samples investigated. However, no recent evidence of the circulation of the virus was found. The seroprevalence rates recorded in these series is low suggesting that Zika virus circulated in Laos but only at a low level at least in Vientiane Capital and Vientiane Province.
Direct Zika virus detection assays in human samples
Direct detection of Zika virus by RT-PCR or virus isolation from blood samples of symptomatic patients remain rare as a consequence of the kinetic of the virus viremia. Indeed, it is now well documented that the peak of viremia precedes the onset of the symptoms and that the level of viremia decline rapidly during the acute phase. To increase the chances of detecting the presence of Zika virus in Vientiane Capital, we retrospectively investigated collections of plasma/serum obtained from asymptomatic blood donors by our colleagues of the Vaccine Preventable Diseases Laboratory at IPL. A first series of 300 samples obtained in 2013 was extracted and tested by Zika virus real time RT-PCR. Among these samples, no positive signal was obtained.
It is now recognized that direct detection of Zika virus in blood samples from patients is poorly sensitive as the viremia precede the acute phase, but also as a consequence of the inconspicuous nature of the symptom onset episode. The discovery of prolonged excretion of West Nile virus in urine from patients contaminated by transfusion or transplantation, brought the attention on this sample as an alternative for the direct diagnosis of arboviruses. Recent studies demonstrated that the duration of Zika virus excretion in urine exceeds lasting days to weeks the phase of production in the blood. The usefulness of urine for the direct diagnosis of dengue has also been proven. Clinicians participating in the IPL passive surveillance network in Vientiane were made aware of the extension of diagnosis capacities and were encouraged to collect a urine sample for each dengue suspected case. A total of 419 urine samples (332 in 2016 and 87 in 2017) were collected from September 2016 to July 2017 and analyzed for the presence of arbovirus markers.
All these samples were tested for the presence of Zika virus RNA by real-time RT-PCR but no positive signal was obtained in this series. Indirect evidence of Zika virus was found in this study.
In addition, differential diagnosis algorithm has been adapted to include Zika virus in the screening of dengue-like syndromes. Thanks to the involvement of the clinicians, appropriate samples (i.e. urine) are now collected in routine offering optimal conditions for Zika virus direct detection.







