Inventory of cave-dwelling hematophagous insects in Laos
 Project coordinator: Dr. Khamsing Vongphayloth; under the supervisor of Dr. Paul Brey, Director, Institut Pasteur du Laos, Vientiane, Lao PDR
Project coordinator: Dr. Khamsing Vongphayloth; under the supervisor of Dr. Paul Brey, Director, Institut Pasteur du Laos, Vientiane, Lao PDR
Staff members:
Khaithong Lakeomany
Nothasine Phommavanh
Phonesavanh Luangamath
Funding support: National Geographic Society:
Exploration Grant
Duration: 1 year (2019)
Background and Relevance
Vector-borne diseases (VBDs) were the second cause of emerging infectious disease (EID) events after zoonotic pathogens (1). Over the past decades, there has been significant emergence and reemergence of several vector-borne diseases such as malaria, leishmaniasis, dengue, Yellow fever, Zika, Chikungunya and plague (2-4). The emergence of new and resurgence of old known vector-borne pathogens can be associated with several factors including adaptation and change of microorganism, habitat changes, globalization, tourism and travel, etc. (5, 6).
Cave ecosystems are specific environmental conditions that provide a suitable place for insects and harbor many different opportunist pathogens, such as viruses, bacteria and fungi (7, 8). Many of these pathogens infect cave-dwelling vertebrates, especially bats (9-13). Some of them could be transmitted from one vertebrate host to another by hematophagous arthropod vectors (e.g. mosquitoes, sandflies, bat flies and biting midges) (14-16). Humans and animals are a risk of exposure to arthropod vectors in caves and in the surrounding areas.
In southeast Asia (SE-Asia), cave visiting is more and more popular for many reasons, such as for resource gathering by the local populations or economic purposes, such as ecotourism or spiritual purposes (cave-dwelling monks). Therefore, such growing human incursion into caves may increase the risk of exposure and spillover of emerging pathogens that circulate among cave-dwelling vertebrates. The recent discovery in humans of the monkey parasite Plasmodium knowlesi stands out as an excellent example of the potential transfers of animal parasites to humans (17, 18). SE-Asia is a known hotspot of biodiversity. However, very little is known about cave-dwelling hematophagous insects that are medically important, such as mosquitoes (Culicidae), sandflies (Phlebotominae) and biting midges (Ceratopogonidae).
To date, eight mosquito species are known to be true troglodytes, two species are known to enter caves, and two others have been found at the entrances of caves (19). Little is known about mosquito species in caves in Laos.
To date, eight mosquito species are known to be true troglodytes, two species are known to enter caves, and two others have been found at the entrances of caves (19). Little is known about mosquito species in caves in Laos.
Before the 1990s, SE-Asia was considered as a region without autochthonous transmission of leishmaniasis hence there is a paucity of knowledge on phlebotomine sandflies. A total of 27 species of sandflies have been identified in Thailand with widespread distribution, high density and diversity inside caves (20-23). Se. gemmea and Se. barraudi, was suspected as the vector of Leishmania siamensis in the southern part of Thailand (24). In Laos, only one cavernicolous species of the sandfly, Chinius genus, was reported by N. Léger et al (25), while two species of the Phlebotomus genus and five species of the Sergentomyia genus were reported by L. Quate (26). From our preliminary results of SandMap project of the Institut Pasteur du Laos (IP-Laos) in 2015 (Khammouane Province), From our preliminary results of SandMap project of the Institut Pasteur du Laos (IP-Laos) in 2015 (Khammouane Province), showed that at least 20 different groups of sandfly females were classified using their morphological characters (unpublished data). Conversely, nothing is known about biting midges in caves in Laos. Sixty-six species of Culicoides were reported in Laos (27). To our knowledge, data on the diversity of cavernicolous insects of medical or veterinary interest are very limited. Moreover, almost nothing is known about their biology, population dynamics and community structure. Thus, to fulfill this gap, we would like to investigate mosquitoes, sandflies and biting midges in caves in Laos. Our hypothesis is that density and species richness of cave-dwelling insects in Laos are high, similar in each cave from different provinces, and related to environmental factors, especially inside humidity, temperature and outside rainfall.
Goals and Objectives
Inventory of Culicidae, Phlebotominae and Ceratopogonidae will help to address the knowledge gap in their diversity, density and population dynamic in caves and areas near caves in Laos in relation with relevant environmental parameters, particularly cave humidity, temperature and external rainfall. The specific objectives are as follows: 1) to describe density and species richness of cave-dwelling insects in Laos, 2) to investigate the cave similarity in terms of species composition and density, and 3) to investigate the relationships between inside humidity, temperature, monthly rainfall (chosen as environmental variables) and the variations of insect density and diversity indices during the study period.
Methodology
Field collections
Mosquitoes, sand flies and biting midges were collected inside caves and outside caves alongside karstic limestone mountains (peri-karstic caves) that are located in the Vientiane Province. Three districts of karstic limestone mountains were selected: Fueng District (18°30’N, 101°59’E), Hin Heup District (18°46’N, 102°16’E), and Vang Vieng District (18°58’N, 102°20’E). Among three districts, Vang Vieng is well known for cave exploration with increasing numbers of tourists visiting each year. It is one of the top tourism destinations in the central part of Laos. To obtain baseline data on the diversity and population dynamics of hematophagous insects in karstic caves and peri-karstic cave areas, two or three caves from each district were selected. One-week surveys were conducted at the selected field sites (Table 1 for more detail).
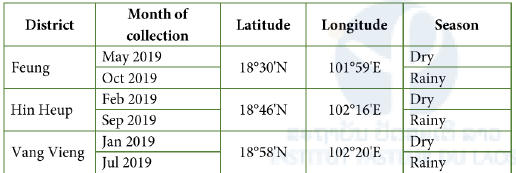
Table 1: Field collection sites and dates
Collection procedure
Collections were conducted two times in each site, during the dry season (approximately November to April) and the rainy season (May to October), to maximize collection yield and observe potential temporal patterns. In each selected location, standard collection methods using CDC light traps were used for hematophagous insect collection inside caves, and near cave areas between 4–6 p.m. and 8–9 a.m for 6-7 nights. Climate data were also recorded by Easy data logger.
All insects were feezed at −20 °C for 20–30 minutes. Specimens were stored at 70% ethanol and then transported to the IP-Laos laboratory in Vientiane capital for morphological identification.
Specimen preparation and identification
Mosquitoes were identified under stereo-microscope using related identification keys (28-33). Mosquito specimens were pinned and deposited at the Insect Arthropod Collection Room of the IP-Laos. For the sandfly and biting midge specimens, head, wings, and abdomen genitalia of both sexes were cut under a stereomicroscope using sterile needles. Head, wings, and genitalia were mounted on slides using PVA mediums and morphological identification under a compound microscope using related morphological identification keys. Sandflies were morphologically identified using dichotomous keys of Lewis and other related references (26, 34, 35). Biting midges were tentatively identified using the keys and illustrations of Ratanaworabhan (36) and Howarth (37). Both sandfly and biting midge sample slides will be provided to Dr. Jerome Depaquit at the Université de Reims Champagne-Ardenne for quality assurance and deposited the Arthropod Collection Room of the IP-Laos.
Preliminary results
The density of Diptera assemblages
A total of 21,518 arthropod hematophagous insects were collected from both inside and outside caves, of which 20,518 were sandflies, 1,216 were mosquitoes and 95 were possibly biting midges. In all sampled districts, sandflies were predominant (Table1) with the apparent density (number of specimens collected per trap and per day) of 118.35 in Fueng, 52.24 in Hin Heup and 22.79 in Vang Vieng. The density of sandflies was slightly higher in the dry season, except in the Vang Vieng district. Overall the density of mosquitoes was low in all districts, with a value of 3.91 and was lower in the dry season than a rainy season (Table 2).
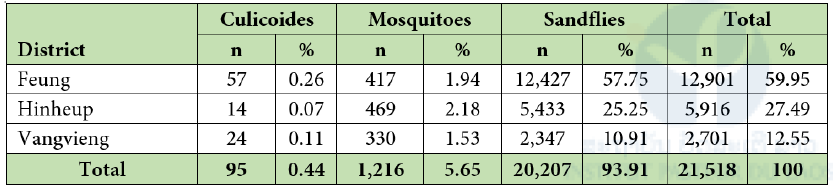
Table 2: Total number of samples collected by the district
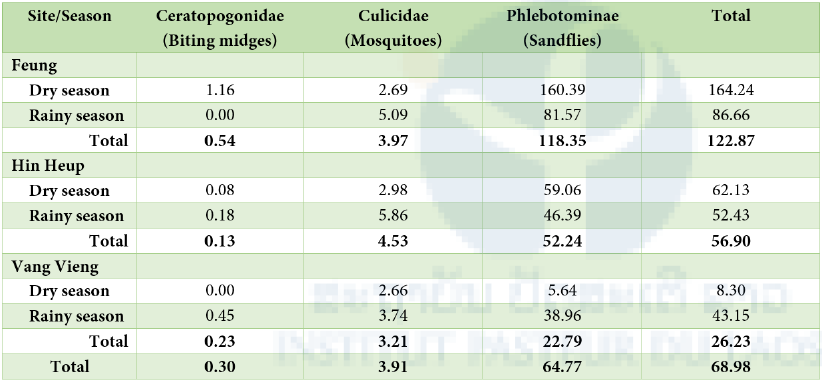
Table 3: Density of arthropod hematophagous insects (number of specimens collected per trap and per day) collected by season
Inventory and diversity of Diptera assemblages
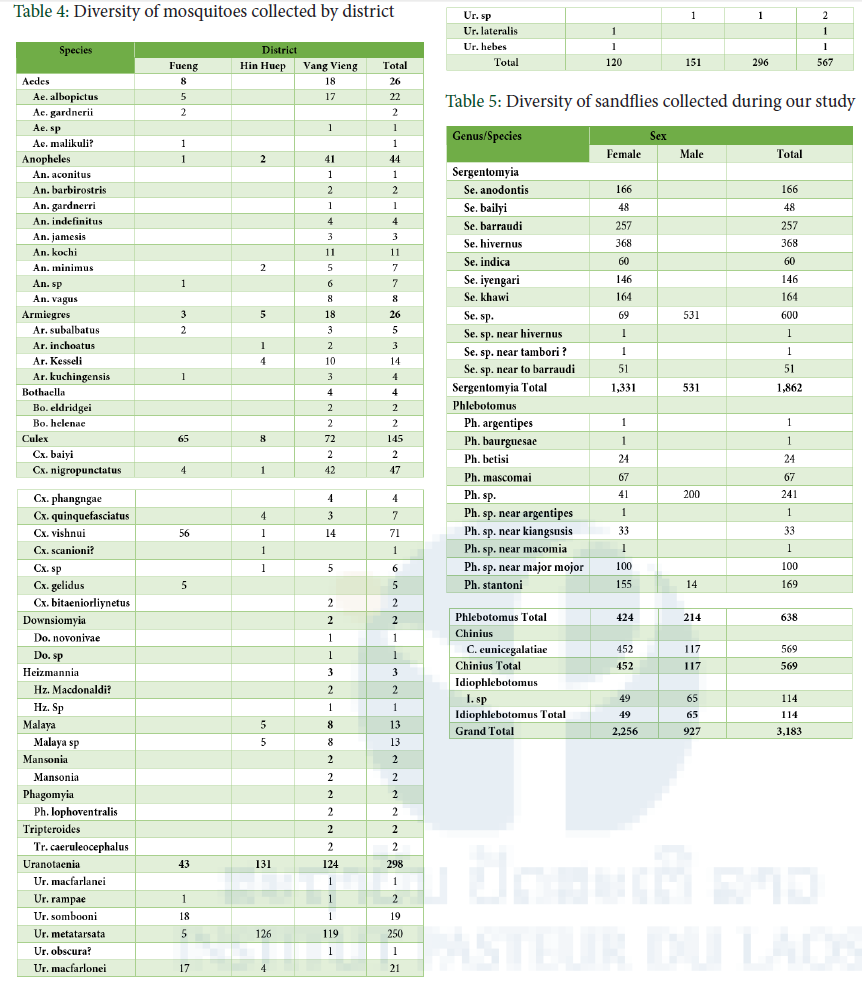
A total of 567 mosquitoes was quickly screen to species so far, around 40 species from 12 genera were suspected identified, many species reported here still need to confirm identification (Table 4). Uranotaenia was the most abundant genus followed by Culex spp.
A total of 3,183 sandflies were cut and morphological identification, at least 17 species were suspected identified from 4 genera: Chinius, Idiophlebotomus, Phlebotomus and Sergentomyia. Sergentomyia was the most abundant genus (1,862/3,183).
Discussion
This is the first study of the cave-dwelling blood-sucking insects in Laos, where the data on the diversity of cavernicolous insects of medical or veterinary interests are very limited. Overall, 21,518 arthropod hematophagous insects were collected from both inside and outside caves, of which 20,518 were sandflies, 1,216 were mosquitoes and 95 were possibly biting midges.
So far, around 40 species from 12 mosquito genera and at least 17 species of 4 sandfly genera: Chinius, Idiophlebotomus, Phlebotomus and Sergentomyia suspected identified. More than 8 species of mosquitoes and sandflies are suspected to be new records for Lao PDR. We will continue to work on identification confirmation. The identification of biting midges is now ongoing. More detail analysis on density and species richness; cave similarity in term of species composition and density; and the relationships between inside humidity, temperature, and monthly rainfall (chosen as environmental variables) and the variations of insect density and diversity indices will be performed and submitted to an international journal (Entomology).
References
1. Jones KE, Patel NG, et al. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990-3.
2. Chan JF, Choi GK, et al. Zika fever and congenital Zika syndrome: An unexpected emerging arboviral disease. The Journal of infection. 2016;72(5):507-24.
3. Gratz NG. Emerging and resurging vector-borne diseases. Annual review of entomology. 1999;44:51-75.
4. Vasconcelos PF, Calisher CH. The emergence of Human Arboviral Diseases in the Americas, 2000-2016. Vector-borne and zoonotic diseases. 2016;16(5):295-301.
5. Harrus S, Baneth G. Drivers for the emergence and re-emergence of vector-borne protozoal and bacterial diseases. International journal for parasitology. 2005;35(11-12):1309-18.
6. Dash AP, Bhatia R, et al. Emerging and re-emerging arboviral diseases in Southeast Asia. Journal of vector-borne diseases. 2013;50(2):77-84.
7. Obame-Nkoghe J, Leroy EM, et al. Diversity and role of cave-dwelling hematophagous insects in pathogen transmission in the Afrotropical region. Emerging microbes & infections. 2017;6(4):e20.
8. Jurado V, Laiz L, et al. Pathogenic and opportunistic microorganisms in caves. Int J Speleol. 2010;39(1):15–24.
9. Konstantinov OK, Diallo SM, et al. [The mammals of Guinea as reservoirs and carriers of arboviruses]. Meditsinskaia parazitologiia i parazitarnye bolezni. 2006(1):34-9.
10. Gomez-Hernandez C, Bento EC, et al. Leishmania infection in bats from a non-endemic region of Leishmaniasis in Brazil. Parasitology. 2017;144(14):1980- 6.
11. Maganga GD, Bourgarel M, et al. Bat distribution size or shape as a determinant of viral richness in African bats. PloS one. 2014;9(6):e100172.
12. McKee CD, Kosoy MY, et al. Diversity and phylogenetic relationships among Bartonella strains from Thai bats. PloS one. 2017;12(7):e0181696.
13. Anh PH, Van Cuong N, et al. Diversity of Bartonella spp. in Bats, Southern Vietnam. Emerging infectious diseases. 2015;21(7):1266-7.
14. Kamani J, Baneth G, et al. Bartonella species in bats (Chiroptera) and bat flies (Nycteribiidae) from Nigeria, West Africa. Vector-borne and zoonotic diseases. 2014;14(9):625-32.
15. Adam JP. [Transmission of Hemosporidia by Anopheles Cavernicolus in the Caves of the Congo (Brazzaville)]. Bulletin of the World Health Organization. 1965;32:598-602.
16. Grard G, Fair JN, et al. A novel rhabdovirus associated with acute hemorrhagic fever in central Africa. PLoS pathogens. 2012;8(9):e1002924.
17. Maeno Y, Culleton R, et al. Plasmodium knowlesi and human malaria parasites in Khan Phu, Vietnam: Gametocyte production in humans and frequent coinfection of mosquitoes. Parasitology. 2017;144(4):527- 35.
18. Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clinical microbiology reviews. 2013;26(2):165-84.
19. Harbach RE, Taai K. Nyctomyia biunguiculata, a new cavernicolous species of tribe Aedini (Diptera: Culicidae) from southern Thailand. Zootaxa. 2014;3895(3):427-32.
20. Apiwathnasorn C, Samung Y, et al. Cavernicolous species of phlebotomine sand flies from Kanchanaburi Province, with an updated species list for Thailand. The Southeast Asian journal of tropical medicine and public health. 2011;42(6):1405-9.
21. Apiwathnasorn C, Sucharit S, et al. A brief survey of phlebotomine sandflies in Thailand. The Southeast Asian journal of tropical medicine and public health. 1989;20(3):429-32.
22. Polseela R, Apiwathnasorn C, et al. Seasonal variation of cave-dwelling phlebotomine sandflies (Diptera: Psychodidae) in Phra Phothisat Cave, Saraburi Province, Thailand. The Southeast Asian journal of tropical medicine and public health. 2007;38(6):1011-5.
23. Polseela R, Depaquit J, et al. Description of Sergentomyia phadangensis n. sp. (Diptera, Psychodidae) of Thailand. Parasites & vectors. 2016;9:21.
24. Chusri S, Thammapalo S, et al. Animal reservoirs and potential vectors of Leishmania siamensis in southern Thailand. The Southeast Asian journal of tropical medicine and public health. 2014;45(1):13-9.
25. Leger N, Depaquit J, et al. Chinius eunicegalatiae n. sp. (Diptera; Psychodidae), a cavernicolous sandfly from Laos. Annals of tropical medicine and parasitology. 2010;104(7):595-600.
26. Quate LW. A REVIEW OF THE INDO-CHINESE PHLEBOTOMINAE. PACIFIC INSECTS. 1962;4(2):251- 67.
27. Howarth FG. Biosystematics of the Culicoides of Laos (Diptera: Ceratopogonidae). International Journal of Entomology. 1985;27:1–96.
28. Rattanarithikul R, Harbach RE, et al. Illustrated keys to the mosquitoes of Thailand. VI. Tribe Aedini. The Southeast Asian journal of tropical medicine and public health. 2010;41 Suppl 1:1-225.
29. Rattanarithikul R, Harbach RE, et al. Illustrated keys to the mosquitoes of Thailand V. Genera Orthopodomyia, Kimia, Malaya, Topomyia, Tripteroides, and Toxorhynchites. The Southeast Asian journal of tropical medicine and public health. 2007;38 Suppl 2:1-65.
30. Rattanarithikul R, Harrison BA, et al. Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. The Southeast Asian journal of tropical medicine and public health. 2006;37 Suppl 2:1-128.
31. Rattanarithikul R, Harrison BA, et al. Illustrated keys to the mosquitoes of Thailand III. Genera Aedeomyia, Ficalbia, Mimomyia, Hodgesia, Coquillettidia, Mansonia, and Uranotaenia. The Southeast Asian journal of tropical medicine and public health. 2006;37 Suppl 1:1-85.
32. Rattanarithikul R, Harbach RE, et al. Illustrated keys to the mosquitoes of Thailand. II. Genera Culex and Lutzia. The Southeast Asian journal of tropical medicine and public health. 2005;36 Suppl 2:1-97.
33. Rattanarithikul R, Harrison BA, et al. Illustrated keys to the mosquitoes of Thailand I. Background; geographic distribution; lists of genera, subgenera, and species; and a key to the genera. The Southeast Asian journal of tropical medicine and public health. 2005;36 Suppl 1:1-80.
34. Lewis DJ. A Taxonomic Review of the Genus Phlebotomus (Diptera, Psychodidae): British Museum (Natural History); 1982.
35. Lewis DJ. The phlebotomine sandflies Diptera: Psychodidae of the Oriental Region1978.
36. Ratanaworabhan NC. An illustrated key for the genera of Ceratopogonidae (Diptera) of the world 1969.
37. Howarth FG. Biosystematics of the Culicoides of Laos (Diptera: Ceratopogonidae). International Journal of Entomology. 1985;27:1-96.
38. Shannon CE. The mathematical theory of communication. 1963. MD computing: computers in medical practice. 1997;14(4):306-17.
39. Chao A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics. 1987;43(4):783-91.
40. Magurran AE. Ecological diversity and its measurement. Princeton, N.J.: Princeton University Press; 1988.
41. Magurran AE. Measuring Biological Diversity New York, NY: John Wiley & Sons; 2013. Available from: http://nbn-resolving.de/ urn:nbn:de:101:1-2014122012826.









