Assessing the risk of insecticide resistance in the tiger mosquito: A predictive approach combining experimental selection and molecular markers (TigeRisk)

Phonesavanh teaches experimental for trainee
Project coordinator: JP David (CNRS, France), Sébastien MARCOMBE (IPL)
Staff members: Phoutmany THAMMAVONG, Phonesavanh LUANGAMATH, Somphat NILAXAY and Vaekey VUNGKYLY
Background
By transmitting several arboviral diseases (dengue, Chikungunya, Zika) Aedes mosquitoes affect public health worldwide including French overseas territories. Since its invasion by the tiger mosquito Aedes albopictus, France metropolitan is now at risk of arbovirus transmission.
Targeting adult mosquitoes with pyrethroid insecticides (PYR) such as deltamethrin constitutes the first line of defense to limit outbreaks. However, mosquitoes have evolved resistance mechanisms to resists insecticides. PYR resistance is widespread in Ae. aegypti and reduces the efficiency of mosquito control in several tropical regions including French overseas territories. Recently resistance has also been reported in populations of the tiger mosquito Ae. albopictus located in various continents including southern Europe and LA Réunion island. Considering its expanding distribution and its growing importance in arbovirus transmission, the rise of PYR resistance in this species represents a major public health concern. In the context of the lack of new insecticides available for public health, managing insecticide resistance is vital until novel control tools are implemented. However, this requires understanding resistance mechanisms and tracking them efficiently in natural populations. Multiple insecticide resistance markers have been identified in Ae. aegypti but barely any are yet available in the tiger mosquito.
In this context, the TigeRisk2 project will break through scientific and technological barriers for characterizing novel DNA markers of PYR resistance in the tiger mosquito Ae. albopictus and track them in natural mosquito populations in order to evaluate the risk of resistance emergence in this species. For achieving this, the consortium will gather 5 complementary teams and combine field mosquito collections with long-term laboratory experiments and state-of-the-art molecular technologies. The detailed objectives of the project are as follows:
1) Sample various mosquito populations worldwide in geographical regions where resistance has been identified or is suspected. Then measure their resistance to PYR and colonize populations suspected to carry resistance alleles in the laboratory.
2) Select these populations with deltamethrin for several generations in order to increase the frequency of resistance alleles.
3) Combine dose-response bioassays and next-generation sequencing for identifying DNA markers of resistance.
4) Design high-throughput diagnostic tests for the best resistance markers.
5) Monitor their frequency in natural populations and evaluate the risk of resistance.
These objectives will be tackled by combining the expertise of two research teams highly recognized in the field of insecticide resistance in mosquitoes (CNRSLECA)
Grenoble and ISEM Montpellier) together with three partners having high expertise in mosquito collections and surveillance (EID-RA, IPL and ARSOI). By positioning
itself upstream the emergence of insecticide resistance in the tiger mosquito, the TigeRisk2 project will ensure the delivery of useful tools for the detection and the management of resistance in this invasive mosquito species.
Activities
Mosquito collection Mosquito
collections were implemented in 4 provinces in 2020 (Figure1; Vientiane Province: 15-21 June; Vientiane Capital: 29 June – 3 July; Khammouane Province: 15-21 July; Borlikhamxay Province: 22- 29 July). Larvae were collected from several types of habitats but mainly in used tires. All mosquitoes from each province were in the pool and reared until adults in the colony room of the institute.
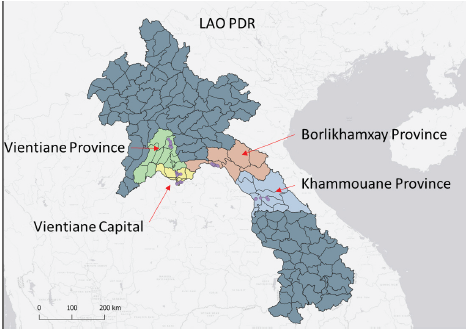
Figure 1. Map of mosquito collection in Lao PDR, 2020.
Morphological mosquito identification
For all the mosquito populations collected, larvae and pupae were reared until adult (F0 generation). After adult identification, the mosquitoes obtained were separated by species. Only Aedes albopictus were kept for breeding. Females mosquitoes were then blood-fed using the Hemotek technic and the eggs obtained were kept for the adult bioassays and the composite population. Approximately 3,000 Aedes albopictus larvae were collected from each study site. Blood feeding of the females obtained started in July to obtain the F1. Thirty males and 30 females of the F0 of each population were kept at -20C in silica gel.
Susceptibility bioassays
Adult bioassays were run using filter papers provided by LECA, treated with a dose of deltamethrin at 0.015% and 0.03%. Mortality resulting from tarsal contact with treated filter papers was measured using WHO test kits against adult mosquitoes of the different populations. Six batches of 25 non-blood-fed females (3–5 days of age, F1) were introduced into holding tubes and maintained for 60 minutes at 27 ± 2°C and relative humidity of 80 ± 10%. Insects were then transferred into the exposure tubes and placed vertically for 60 minutes under subdued light. Mortality was recorded 24 hours after exposure.
Composite population The Aedes albopictus composite population is currently being established.
Results
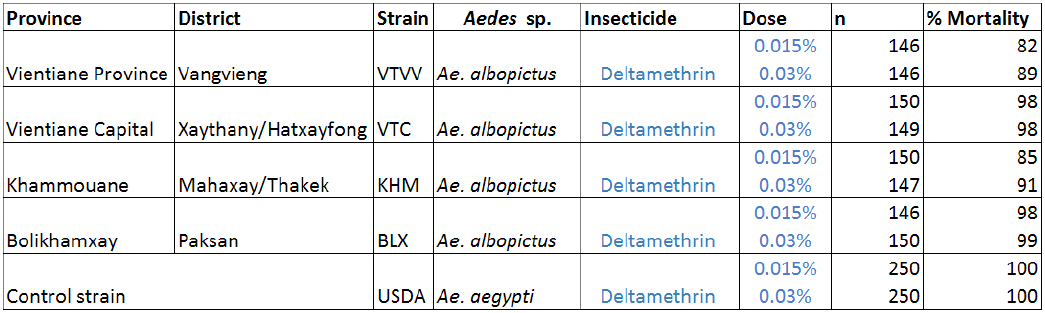
Results are presented in Table 2. The mortalities of the different Ae. albopictus varied from 82 to 98% and 85 to 99% at the dose of 0.015 and 0.03%. The results with the 0.03% WHO diagnostic dose indicate that the VTVV population is resistant to deltamethrin and the other populations present reduced susceptibility to this pyrethroid (suspected resistance).
Table2. Insecticide resistance bioassay on adult Ae. albopictus from Laos