Assessing the risk of insecticide resistance in the tiger mosquito: A predictive approach combining experimental selection and molecular markers (TigeRisk2)
 Project coordinator:JP David (CNRS, France), Sebastien Marcombe (IPL)
Project coordinator:JP David (CNRS, France), Sebastien Marcombe (IPL)
Staff members: Phoutmany Thammavong, Phonsavanh Luangamath, Somphat. Nilaxay and Vaeky Vungkyly
Context and Objectives
By transmitting several arboviral diseases (dengue, Chikungunya, Zika, …) Aedes mosquitoes affect public health worldwide including French overseas territories. Since its invasion by the tiger mosquito Aedes albopictus, France metropolitan is now at risk of arbovirus transmission.
Targeting adult mosquitoes with pyrethroid insecticides (PYR) such as deltamethrin constitutes the first line of defense to limit outbreaks. However, mosquitoes have evolved resistance mechanisms to resist insecticides.
PYR resistance is widespread in Ae. aegypti and reduces the efficiency of mosquito control in several tropical regions including French overseas territories. Recently resistance has also been reported in populations of the tiger mosquito Ae. albopictus is located in various continents including southern Europe and La Réunion island. Considering its expanding distribution and its growing importance in arbovirus transmission, the rise of PYR resistance in this species represents a major public health concern.
In the context of the lack of new insecticides available for public health, managing insecticide resistance is vital until novel control tools are implemented. However, this requires understanding resistance mechanisms and tracking them efficiently in natural populations. Multiple insecticide resistance markers have been identified in Ae. aegypti but barely any are yet available in the tiger mosquito.
In this context, the TigeRisk2 project will break through scientific and technological barriers for characterizing novel DNA markers of PYR resistance in the tiger mosquito Ae. albopictus and track them in natural mosquito populations in order to evaluate the risk of resistance emergence in this species. For achieving this, the consortium will gather 5 complementary teams and combine field mosquito collections with long-term laboratory experiments and state-of-the-art molecular technologies.
These objectives will be tackled by combining the expertise of two research teams highly recognized in the field of insecticide resistance in mosquitoes (CNRSLECA Grenoble and ISEM Montpellier) together with three partners having high expertise in mosquito collections and surveillance (EID-RA, IPL and ARSOI). By positioning itself upstream the emergence of insecticide resistance in the tiger mosquito, the TigeRisk2 project will ensure the delivery of useful tools for the detection and the management of resistance in this invasive mosquito species.
Methods
Mosquito collections locations and dates
In Laos, during the rainy season 2020, Aedes sp. larvae and pupae were collected in 4 provinces including 6 different districts in more than 40 villages (Figure 1). All the collection sites were geo-referenced. In Cambodia, other collections were made in 2 provinces in September 2020 in Phnom Penh and Kampot. As Ae. albopictus lives in more rural or semi-rural areas or in the forest, for the collections, we selected locations surrounding the city or villages rural areas and/or close to forests. Larvae were collected in several types of breeding habitats such as buckets, flowerpots, household utensils and other containers but most of the time in abandoned tires. After collection, the biological material was brought back to the laboratory at the Institut Pasteur du Laos for rearing and identification. Approximately 20,000 Aedes sp. larvae were collected from all the study sites. Table 1 shows the list of populations reared at the laboratory and used for the bioassays. In 2021, mosquitoes were collected in the same areas as 2020 but only in two provinces (Vientiane capital and V. province) due to the shutdown of provincial boundaries implemented against COVID-19. Similarly, in Cambodia, it was not possible to collect mosquitoes.
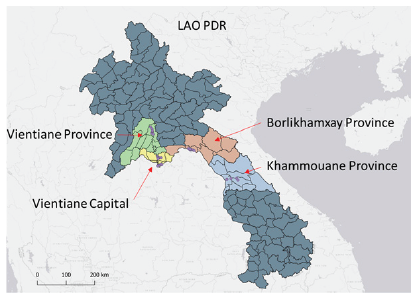
Figure 1. Locations of mosquito collections in Lao
PDR, 2020/2021.
Morphological mosquito identification
For all the mosquito populations collected, larvae and pupae were reared until adult (F0 generation). After adult identification, the mosquitoes obtained were separated by species. There was a great proportion of Ae. aegypti and Armigeres sp. found in the collected samples. Only Aedes albopictus were kept for breeding. Females mosquitoes were then blood-fed using the Hemotek membrane feeding system and the eggs obtained were kept for the adult bioassays and the composite population composition. For each population, 30 males and 30 females were kept at -20°C in silica gel. We received eggs from Cambodia in October 2020 but unfortunately, after emergence, most of the individuals were identified as Ae. aegypti mosquitoes resulting in a very low number of Ae. albopictus. This number (<200) was not enough to implement both the bioassays and the composite population. We chose to use the Ae. albopictus mosquitoes for the composite population.
Susceptibility bioassays
For the bioassays, all the Ae. albopictus individuals from a single province were mixed together to obtain a sufficient number of mosquitoes to implement the bioassays and the creation of the composite population. Adult bioassays were run against the four different mosquito populations from Laos using filter papers provided by LECA, treated with a dose of deltamethrin at 0.015% and 0.03%. Mortality resulting from tarsal contact with treated filter papers was measured using WHO test kits against adult mosquitoes of the different populations. Six batches of 25 non-bloodfed females (3–5 days of age, F1) were introduced into holding tubes and maintained for 60 minutes at 27 ± 2°C and relative humidity of 80 ± 10%. Insects were then transferred into the exposure tubes and placed vertically for 60 minutes under subdued light. Mortality was recorded 24 hours after exposure.
Composite population (CP)
The Aedes albopictus composite population was established at the end of the year 2020 using the mosquito populations for each geographic area sampled from Laos and Cambodia.
At least 1,000 founding individuals from each natural population (except Cambodia) were used to maximize the genetic diversity of composite populations before initiating the selection process for resistant alleles of pyrethroids. For each population, male and female mosquitoes (500 individuals) were separated into different cages just after emergence and were mixed at the same time in a bigger cage when they were about 3-5 days old. After mating, the female was blood-fed. The next generation was then used for the selection.
Selections
For the selection, males and females of the CP were separated just after emergence and were exposed to dose-response bioassays to evaluate the Lethal Dose for 75% (LD 75) with deltamethrin. However, looking at the difficulty encountered to breed Ae. albopictus compared to Ae. aegypti in our laboratory (low fecundity, fertility and low blood-feeding rates), we decided to use the LD50 to ensure to avoid the loss of the CP during the process. The CP was then selected with deltamethrin separately on males and females to generate a resistant strain. The survivors (>500) male and female were mixed for mating and bred until the next generation. The selection was made at every generation. In parallel, the CP was maintained without selection to be used as a reference for molecular analysis. Resistance levels of the 2 strains, selected (CPR) and non-selected (CP-Ns) were implemented every 2 selections. For both strains, 30 males and 30 females were kept in the freezer.
Results
Insecticide resistance status of the field-collected population in Laos Results are presented in Table 2 and Figure 2. The mortalities of the different Ae. albopictus populations from Laos varied from 82 to 98% and 85 to 99% at the dose of 0.015 and 0.03% respectively. The results with the 0.03% WHO diagnostic dose indicate that the VTVV population was resistant to deltamethrin and the other populations presented reduced susceptibility to this pyrethroid insecticide (suspected resistance).
Table2. Insecticide resistance bioassay on adult Ae. albopictus from Laos
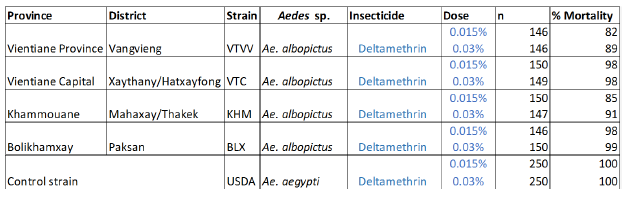
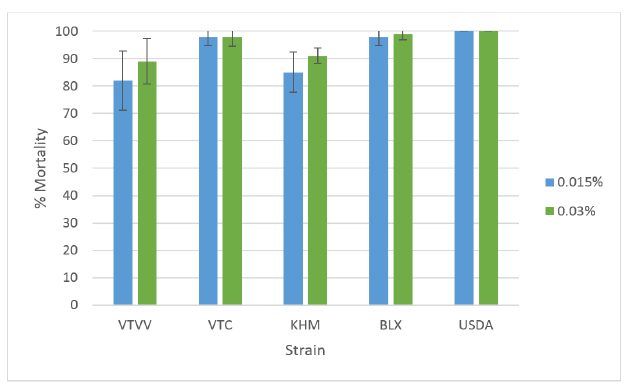
Figure 2. Mortality and SE of Ae. albopictus populations against deltamethrin at different doses (0.015% and0.03%), USDA, Ae. aegypti control susceptible strain.
Mortalities measured during selection of the Composite population
Four selection tests were implemented during the reporting period. For each selections, females of the CP were exposed to the LD50 times determined during the preliminary tests (20 to 23min) but only showed 26 to 29% mortality. Males of the Laos CP showed mortality with an exposure time of 15min between 43 to 60%. Figure 3 shows the mortality of the CP at each selection tests for male and female adults. The results showed that the exposure times should be increased for the female to select more pyrethroid resistant alleles.
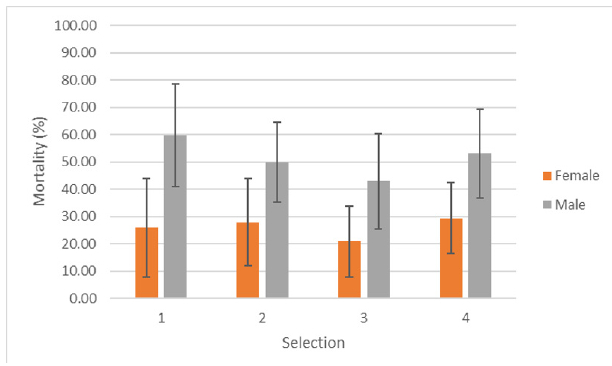
Figure 3. Mortality (%) observed during selection with deltamethrin (0.015%) of the female and male mosquitoesof the CP of Laos with Standard Errors
Insecticide resistance status of the Composite Population of Laos, CP-R and CP-Ns
The results are presented in the table 4. Female mosquitoes were exposed to the different insecticides during 1 hour.
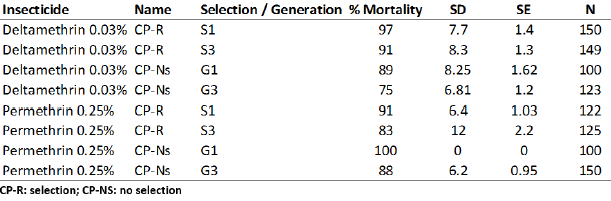
Bioassays on the CP-R and CP-Ns against the different insecticides are presented in Figure 4. The mortality of the CP-R selection 3 against deltamethrin (0.03%) is lower than CP-R after the first selection. For both selection stages mortality is lower with CP-Ns.
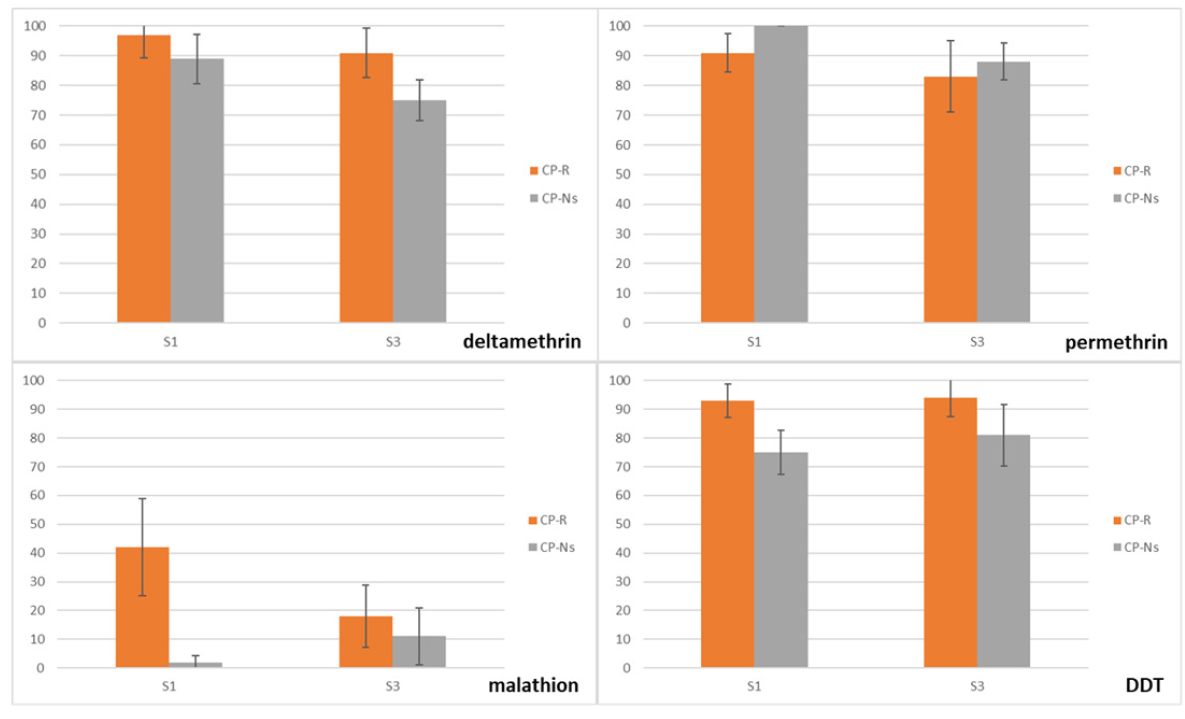
Figure 4. Mortality (%) of the CP-R and CP-Ns after deltamethrin selection tests.
Next step
cThe collections, insecticide resistance tests and selections are completed. The meta-population composed in the laboratory and the dead and alive mosquitoes resulting from the bioassay will be sent to France at the CNRS. They will be used to combine dose-response bioassays and with the next-generation sequencing to identify DNA markers of pyrethroid resistance. The results will help to design high-throughput diagnostic tests for the best resistance markers in the tiger mosquito Ae. albopictus to track them in natural mosquito populations in order to evaluate the risk of resistance emergence in this species.







