Research on mosquitoes, sandflies and their related pathogens in Laos
 Collaboration
Collaboration
• U.S. Naval Medical Research Unit-2.
• Pathogen Discovery Laboratory, IP-Paris-France.
Funding
• U.S. Naval Medical Research Center-Asia (NMRC-A) in support of the Department of Defense Global Emerging Infections Surveillance and Response System (DoD-GEIS).
• French Ministry of Higher Education, Research (MESRI)-IP-Laos
• Fondation Institut Pasteur du Laos (Young Research Grant Challenge 2023).
Objectives
• Local capacity building in medical entomology and vector-borne diseases.
• Inventory of vector species in Laos.
• Pathogen detection from mosquitoes and sandflies in Laos.
Background
Lao PDR is known for the diversity of its landscapes, ecosystems and mountainous that attracted many tourists each year. This developing country located in the middle of the Indochinese peninsula shares borders with many countries and is a hot spot for biodiversity and potential emergence of infectious diseases. Among emerging arboviruses, dengue viruses (DENV) and Chikungunya (CHIKV) are good examples of zoonotic diseases. These arboviruses had sylvatic transmission cycles where they circulated between vertebrate animals and forest dwelling insects before their spill-over into the human population. In a particular environmental condition, some pathogens could cross the species barriers and cause the emergence of new zoonotic diseases. Hence, the associated health risks in a specific ecosystem need to be addressed, particularly those related to potential zoonotic pathogens that circulate among karstic and cave-dwelling vertebrates. Very little is known about hematophagous insects and ectoparasites that are medically important and the associated health risks in the Indochinese peninsula as well as in Laos.
To fill this gap, we decided to pursue our previous work and further investigate the arboviruses in Laos, by carrying out arbovirus studies in hematophagous insects, particularly mosquitoes and sandflies from the karstic areas of Laos.
Methodology
Mosquito and Sandfly sample collection.
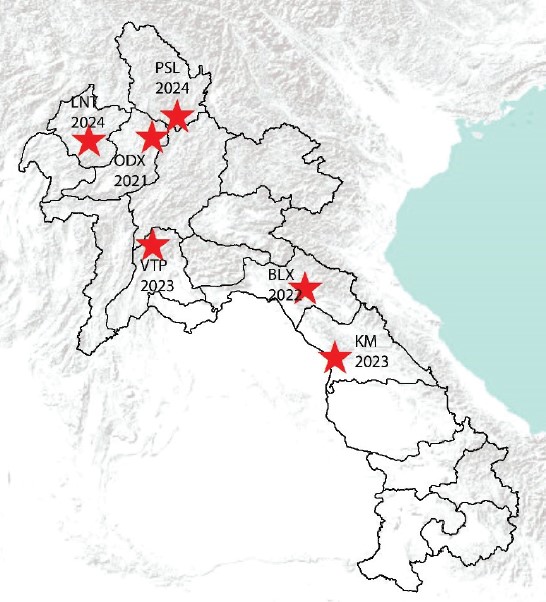
Standard collection methods using CDC light traps were used for sandfly capture from different types of habitats. Traps were set between 4–6 p.m. and 8–9 a.m. Specimens from the IP-Laos repository collected between 2021 and 2024 were used for this study. Mosquitoes and sandflies were collected from 6 provinces including Bolikhamxay, Khammouane, Luangnamtha, Oudomxay, Phongsaly and Vientiane province (Fig. 1).
Figure 1: Map showing locations (red stars) of Mosquito and sandfly collection sites used in this study. Numbesr indicate years of collection. BXL: Bolikhamxay, KM: Khammouane, LNT: Luangnamtha, ODX: Oudomxay, PSL: Phongsaly, and VTP: Vientiane province.
Specimen preparation and identification.
The samples were frozen at −20 °C then sorted and counted. After that, they were kept in an Eppendorf tube with silica gel. Mosquitoes were identified under a stereomicroscope using related identification keys 1-6. Sandfly’s head, wings, and abdomen genitalia of both sexes were cut under a stereomicroscope using sterile needles. Head, wings, and genitalia were mounted on slides using a PVA or Euparal mounting medium. Morphological identification was done under a compound microscope using related morphological identification keys 7,8.
Total nucleic acids extraction.
Individual thorax/whole body in a NucleoSpin® 8 tube was filled with 500 μl of Phosphate-Buffered Saline (PBS) 1X and Lysing Matrix E beads or A (MP Biomedicals). A TissueLyser II system (Qiagen) was used for homogenization for 10 min at a vibration frequency of 25/s. To obtain total nucleic acids extraction (DNA/ RNA) for pathogen screening, a total of 100 μl of each tube was extracted using a NucleoSpin® 8 Virus extraction kit following the manufacturer’s protocol. The remaining 400 μl of each pool was stored at –80°C for further pathogen isolation.
Arbovirus screening.
Mosquitoes and sandflies were screened for the detection of phleboviruses, flaviviruses, and alphaviruses by conventional nested RT-PCR.
Next generation Sequencing.
RNA library construction, and NGS sequencing.
In brief, the quality and concentration of extracted samples were evaluated using Agilent TapeStation and Qubit 2.0 Fluorometer (Invitrogen, USA) respectively. Total RNA libraries were constructed using the SMARTer Stranded Total RNA-seq Kit v3-Pico input mammalian (TaKaRa).
DNA library quality and concentration were evaluated using the same techniques mentioned above. Then the libraries were sent to Macrogen Asia Pacific Pte. Ltd. for NGS using the NovaSeq6000 platform at 20Gb/sample.
Bioinformatics pipeline.
The pathogen discovery lab (IP Paris) team has designed software called Microseek. It is a pipeline oriented initially for pathogen discovery but that can also efficiently detect already known viruses (see https://pubmed.ncbi.nlm. nih.gov/36146797/).
Preliminary results
Mosquito and sandfly species and abundance.
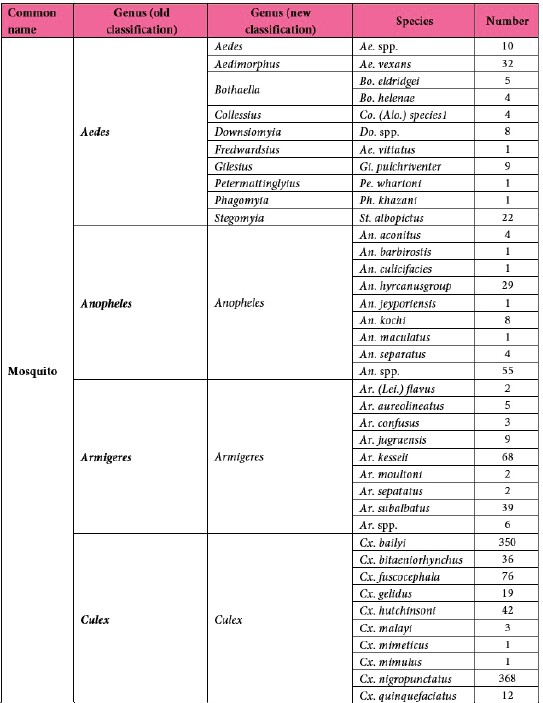
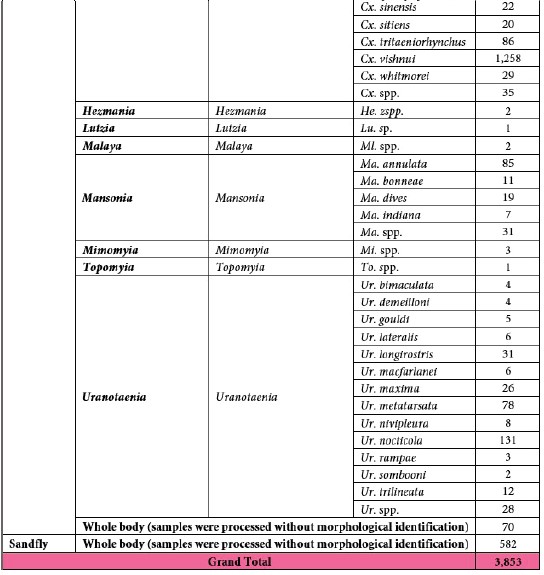
A total of 3,859 samples, including 3,277 mosquitoes and 582 sandflies, were collected from six provinces of Laos between 2019 and 2024 (Fig. 2) and processed in this study. Of these, 3,853 samples underwent morphological identification and sample preparation for laboratory screening. The mosquitoes were classified into 69 species across 11 genera (based on the old classification) (Tab. 8).
Figure 2: Numbers of mosquitoes and sandflies from different provinces used in this study.
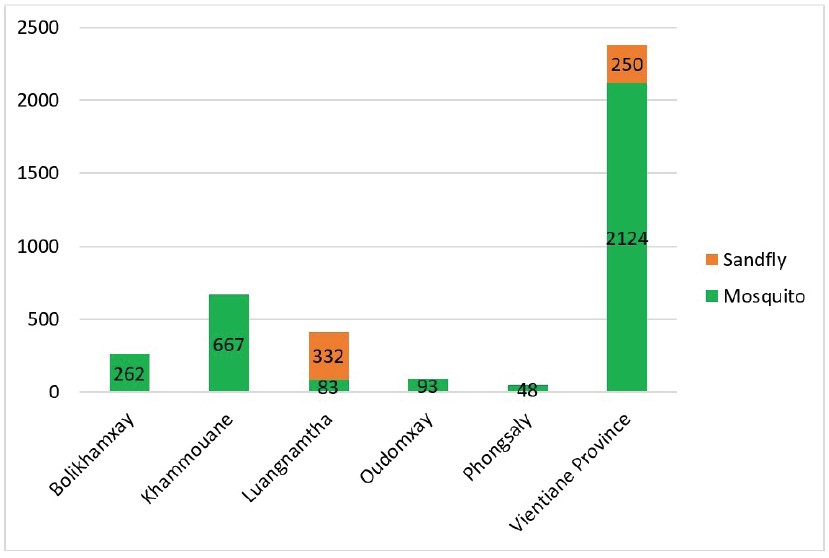
Table 1: Mosquito species composition.
Pan-flaviviruses and pan-phleboviruses screening by RT-PCR.
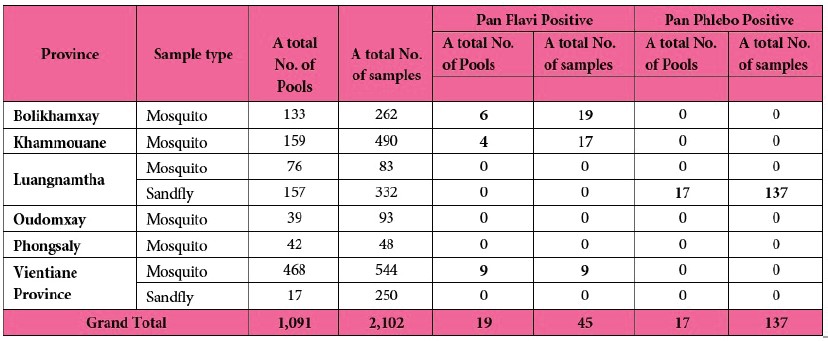
A total of 1,091 pools containing 2,102 samples were screened for flaviviruses and phleboviruses. Nineteen mosquito pools tested positive for pan-flaviviruses, and 17 sandfly pools tested positive for phleboviruses (see Tab. 9 for details).
Table 9: Mosquitoes and sandflies positive for flaviviruses and phleboviruses in this study.
NGS strategy.
In order to characterize flaviviruses from mosquitoes and phleboviruses from sandflies, so far the NGS library of 16 samples of flavivirus-positive mosquitoes and of 6 samples of phlebovirus-positive sandflies was prepared.
Conclusion and perspectives
This study highlights the diversity and abundance of mosquito and sandfly populations across six provinces of Laos, collected between 2021 and 2024. Morphological identification of mosquitoes revealed 69 mosquito species across 11 genera, underlining the rich biodiversity of these mosquito species. Laboratory screening by RTPCR detected the presence of flaviviruses in mosquito pools and phleboviruses in sandfly pools, indicating the circulation of these arboviruses among vectors in Laos.
Future work will focus on completing the NGS sequencing and analysis to identify and characterize flaviviruses and phleboviruses in more detail. This will provide insights into the genetic composition, evolutionary relationships, and potential transmission dynamics of these viruses in Laos.
Furthermore, expanded sampling efforts and enhanced molecular screening in other provinces are recommended to better understand the distribution of vector species and associated viruses. These findings will contribute to the development of targeted vector surveillance and control strategies, aiding in the prevention of arboviral outbreaks in the region.
References
1. Rattanarithikul R, Harrison BA, et al. Illustrated keys to the mosquitoes of Thailand I. Background; geographic distribution; lists of genera, subgenera, and species; and a key to the genera. Southeast Asian J. Trop. Med. public Heal. 36 Suppl 1, 1–80 (2005).
2. Rattanarithikul R, Harbach RE, et al. Illustrated keys to the mosquitoes of Thailand. II. Genera Culex and Lutzia. Southeast Asian J. Trop. Med. Public Health 36 Suppl 2, 1–97 (2005).
3. Rattanarithikul R, Harrison BA, et al. Illustrated keys to the mosquitoes of Thailand III. Genera Aedeomyia, Ficalbia, Mimomyia, Hodgesia, Coquillettidia, Mansonia, and Uranotaenia. Southeast Asian J. Trop. Med. Public Health 37 Suppl 1, 1–85 (2006).
4. Rattanarithikul R, Harrison BA, et al. Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian J. Trop. Med. Public Health 37 Suppl 2, 1–128 (2006).
5. Rattanarithikul R, Harbach RE, et al. Illustrated keys to the mosquitoes of Thailand V. Genera Orthopodomyia, Kimia, Malaya, Topomyia, Tripteroides, and Toxorhynchites. Southeast Asian J. Trop. Med. public Heal. 38 Suppl 2, 1–65 (2007).
6. Rattanarithikul, R. et al. Illustrated keys to the mosquitoes of Thailand. VI. Tribe Aedini. Southeast Asian J. Trop. Med. Public Health 41 Suppl 1, 1–225 (2010).
7. Lewis, D. J. Lewis, D J. 1982. “A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae).” Bulletin of the British Museum (Natural History) Entomology 45, 121–209. Bulletin of the British Museum (Natural History) Entomology vol. 45 121–209. 8. Quate LW. A REVIEW OF THE INDO-CHINESE PHLEBOTOMINAE ( Diptera : Psychodidae ). 4, (1962).
9. Sánchez-Seco, M. P. et al. Detection and identification of Toscana and other phleboviruses by RT-nested-PCR assays with degenerated primers. J. Med. Virol. 71, 140– 149 (2003).
10. Sánchez-Seco, M. P. et al. Generic RT-nested-PCR for detection of flaviviruses using degenerated primers and internal control followed by sequencing for specific identification. J. Virol. Methods 126, 101–109 (2005).
11. Sánchez-Seco MP, Rosario D, Quiroz E, Guzmán G, Tenorio A. A generic nested-RT-PCR followed by sequencing for detection and identification of members of the alphavirus genus. J Virol Methods. 2001 Jun;95(1- 2):153-61.
Publications in 2024
Vongphayloth, K., F. J. Randrianambinintsoa, K. Lakeomany, N. Phommavanh, T. Pongsanarm, V. Vungkyly, P. Luangamath, S. Chonephetsarath, P. T. Brey, and J. Depaquit. 2024. A study on the diversity of phlebotomine sand flies (Diptera, Psychodidae) in karstic limestone areas in Vientiane Province, Laos, with a description of two new species of Sergentomyia Franca and & Parrot. Parasit Vectors 17: 385. Kyoko Sawabe, Tenzin Wangdi, Pradya Somboon, Vongphayloth Khamsing, Moritoshi Iwagami, Siew Hwa Tan, Khatanbaatar Igori, Basu Dev Pandey, Kouichi Morita, Jiamei Sun, Astri Nur Faizah, Yusuf Ozbel, Tran Vu Phong, Vu Sinh Nam, Hwa-Jen Teng, Han-Hsuan Chung, Pai-Shan Chiang, and Shiu-Ling Chen. Special Topics from Asian Countries. In: Kyoko Sawabe, Chizu Sanjoba, Yukiko Higa, editors. Medical Entomology in Asia. Springer Nature Singapore Pte Ltd; 2024. p. 369.
Congress
Oral presentations.
Vongphayloth Khamsing, Randrianambinintsoa José Fano, Lakeomany Khaithong, Phommavanh Nothasine, Pongsanarm Tavun, Vungkyly Veaky, Luangamath Phonesavanh, Chonephetsarath Somsanith, Brey Paul, Buchy Philippe, Jérôme Depaquit. Analysis of phlebotomine sandflies (Diptera: Psychodidae) in Laos from 2012-2024 identifies new species and diverse taxa. In ISOPS XI (International Symposium on Phlebotomine Sandflies XI) 9-13 September 2024. Portorož, Slovenia.
Training activities
Training is undertaken by the team.
1. Basics of bioinformatics and LIPS (Luciferase ImmunoPrecipitation System), PIMES project.







