Investigating hepatitis B virus infection in Lao blood donors: Genotype patterns, occult infection status, prevalence trends and vaccination impact
 Members of the LaoLuxLab / Vaccine-Preventable Diseases laboratory provided training in sample and data collection and sample processing practices for blood bank staff across 18 provinces, at the National Blood Transfusion Center in Vientiane (Lao Red Cross).
Members of the LaoLuxLab / Vaccine-Preventable Diseases laboratory provided training in sample and data collection and sample processing practices for blood bank staff across 18 provinces, at the National Blood Transfusion Center in Vientiane (Lao Red Cross).
Collaboration
• National Blood Transfusion Center, Lao Red Cross.
• Provincial Blood Transfusion Centers.
Funding
• Luxembourg Ministry of Foreign and European Affairs, Defence, Development Cooperation and Foreign Trade.
• Luxembourg Institute of Health.
Objectives
The objectives are to determine the prevalence of occult hepatitis B virus (HBV) infection in Lao blood donors, the geographical HBV genotype distribution in hepatitis B surface antigen (HBsAg) positive donors, the prevalence of HBsAg in repeat donors and the impact of HBV vaccination in donors born after vaccine introduction. We aim to extend the investigation to other blood-borne pathogens, including hepatitis D virus and hepatitis C virus.
Background
Hepatitis B is endemic in Laos, with approximately 8 to 10% of adults being chronically infected. The high endemicity of HBV infection is thought to be a major cause of the high prevalence of liver cirrhosis, liver disease and liver cancer in the nation. Vaccination against hepatitis B was introduced in the country in 2001, but overall vaccination rates with the hepatitis B birth dose and the hepatitis B containing vaccine remain low at 70% and 84%, respectively.
A geographic variation in HBV epidemiology was found in blood donors, with higher exposure and active infection rates in the North of the country, without any apparent explanation for this difference. The study also showed an alarmingly high prevalence of HBsAg, a marker for active infection, in repeat donors. The fact that chronically infected donors seem to return for blood donation warrants a thorough investigation of current blood screening, record keeping, and donor identification practices.
Furthermore, although genotypes B and C are thought to predominate in Laos, the rarer genotype I has also been detected. Importantly, different genotypes and variants have been associated with various degrees of disease severity and resistance to anti-viral medication. Since current data on the genetic composition of HBV strains are lacking for most parts of Laos, nationwide investigations are warranted, also to further elucidate transmission patterns.
A study from 2006 found that 3.9% of donated blood was HBV DNA positive, despite being HBsAg negative, and therefore passed the screening process. Such donated blood (occult HBV infected; OBI) may represent a risk of infection to transfusion recipients. Due to financial and logistical constraints, the Lao Red Cross has not been able to invest in automated PCR screening of donated blood, which would detect OBI infections.
To address the current knowledge gaps, we proposed a follow-up study in collaboration with the Lao Red Cross to investigate the prevalence of OBI in Lao blood donors, the geographical HBV genotype distribution, the prevalence of HBsAg in repeat donors and the impact of HBV vaccination on donors born after vaccine introduction.
Methodology
In collaboration with the Lao National Blood Transfusion Center (NBTC), a cross-sectional study is currently being conducted, aiming to collect a total of about 6300 samples from blood donors in all 18 provinces. Participants are recruited when coming to a provincial donation center for blood donation. The blood donation center staff manages participant enrolment, including providing study information, obtaining informed consent and recording information on age, sex and risk factors. After enrolment, participants are asked to provide a blood sample for laboratory analysis during blood donation.
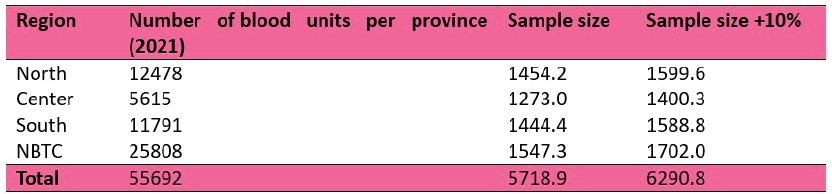
For the calculation of the target sample size, the provinces in the country were grouped into three regions North (Bokeo, Huaphan, Luang Namtha, Luang Prabang, Oudomxay, Pongsaly, Xayaboury and Xieng Khuang), Center (Bolikhamxay, Vientiane Province and Xaysomboun) and South (Attapeu, Champasack, Khammuane, Saravan, Savannakhet and Sekong). The NBTC was regarded as an additional study location rather than grouping with the provinces in the Center due to its high number of yearly blood donations. We used an expected proportion of 3.9% OBI for our calculations based on a previous study, and the number of blood donation units per province in 2021 as a reference for the population size.
The target sample size was calculated by region and for the NBTC considering a potential participant withdrawal rate of 10% (Table 1).
Table 1. Sample size estimation per region.
From each participant, 5 ml of whole blood was collected. The serum was separated and stored at the blood banks at -20°C until transport to NBTC. After transferral to the Institut Pasteur du Laos, the samples were stored at -80°C.
Serological markers for HBV will be tested by commercially available Enzyme-Linked Immunosorbent Assay kits. Samples will also be tested for the presence of HBV DNA by PCR. In addition to HBV, we plan to extend the investigation to other blood-borne pathogens, including hepatitis D virus and hepatitis C virus.
Results
The study is ongoing. The sample collection is nearly completed, with a cumulative 6200 samples from all provinces, except for Phongsaly. Currently, we are planning the laboratory testing.
Conclusion & perspectives
Investigating the geographical distribution of HBV genotypes, the prevalence of OBI infection, the HBsAg prevalence in repeat donors and the impact of HBV vaccination in Laos is critical for tailoring public health strategies. This study will improve our understanding of the current state of HBV epidemiology all over Laos and provide valuable information for the development of targeted interventions and resource allocation.







