Maternal and neonatal vaccination protection in the Lao PDR
Blood sample collection from an umbilical cord of a neonate and subsequent sample processing.
Collaboration
• Mahosot hospital in Vientiane.
• Provincial hospitals.
Funding
• Luxembourg Ministry of Foreign and European Affairs, Defence, Development Cooperation and Foreign Trade.
• Luxembourg Institute of Health.
Objectives
The aim of this study is to investigate antibody prevalence and titres against several vaccine-preventable diseases in women giving birth and their new-borns in different regions in Laos. Data on vaccination and infection history of the participants will be used to analyse the factors impacting the effectiveness of transplacental transfer of antibodies.
Background
Maternal antibodies are transferred across the placenta to the unborn child in utero and can protect infants against infectious diseases for the first few months of life. These maternal antibodies may have been acquired by vaccination or previous infection of the mother.
The current infant vaccination schedule in Laos includes pentavalent vaccine (diphtheria, tetanus, pertussis, hepatitis B, Haemophilus influenzae type b) administered at the age of 6,10 and 14 weeks, and measles/rubella vaccine administered at 9-11 and 12-23 months of age. Nevertheless, in Laos, there are frequent outbreaks of measles, rubella, diphtheria and other vaccine preventable diseases, which can affect infants at any age before they are vaccinated, when maternal antibodies should provide protection. In many districts of Laos, there is low or incomplete vaccination coverage, and therefore lack of early waning of maternally acquired antibodies may leave infants susceptible to infection for longer periods.
Sufficiently high maternal antibody concentrations are essential for the protection of infants after birth and during their first few months of life. However, the titer of transferred antibodies varies between individuals. We designed a study to investigate the antibody prevalence and titers against vaccine-preventable diseases in women giving birth and their new-borns in different regions in Laos.
We plan to analyse the vaccination and infection history of the women and also any factors that may impact the transplacental transfer of antibodies. The data that will be generated for this study may be used to improve maternal and infant health in the country. The study will provide evidence-based data to the Lao Ministry of Health and the National Immunization Programme.
Methodology
This is a cross-sectional study on antibody prevalence and levels against vaccine-preventable diseases in women and their new-borns in Laos. A total of 400 samples were collected from each participating provincial hospital, resulting in a sample size of 1600 mother and new-born pairs. The participating hospitals are located in Vientiane Capital, Vientiane Province, Bolikhamxay, Xaysomboun, Khammuane, Luang Prabang, Huaphan and Saravan Province (Figure 1). These locations were chosen to obtain a broad geographical distribution in the country.
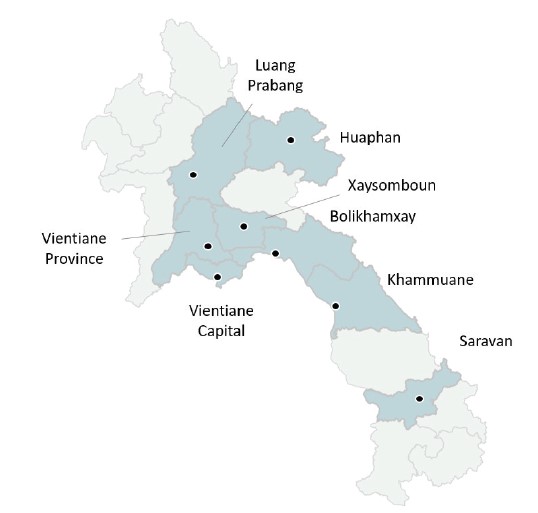
Figure 1. Map of study locations. Black dots denote provincial capitals.
Pregnant women who were admitted to the hospital for delivery were invited to take part in the study by the hospital staff. After informed consent, a 5 ml venous blood sample was taken from each woman by a qualified healthcare worker. In order to capture demographic information and other relevant factors, such as vaccination status and infection history, a short face-to-face interview was conducted. Following delivery, a 5 ml blood sample was taken from the umbilical cord. The samples were sent to the laboratory of the respective hospital for further processing and serum separation. The serum was stored at the hospital at -20°C until transfer to the Institut Pasteur du Laos. Currently, the samples are kept at -80°C.
The samples will be tested for antibodies against multiple vaccine-preventable diseases, such as pertussis, measles, tetanus, diphtheria and rubella, by using commercially available enzyme-linked immunosorbent assay kits.
Results
The study is ongoing. We received all samples from 1600 mother and new-born pairs from the eight targeted provinces. The laboratory analyses are planned for the beginning of January 2025.
Conclusion & perspectives
The results of this study will provide essential insights into the presence and titers of maternal antibodies and the success of vaccination programs in diverse settings within the country. By identifying regional disparities and influencing factors, the research can guide public health decision makers to further improve mother and child health and to reduce infant and maternal morbidity and mortality rates.







