Deployment of an integrated vector-biology and pathogen discovery platform in the Lao PDR (BioLao2)

Supervisors:
+ Dr. Sebastien Marcombe (IPL)
+ Dr. Marc Grandadam (IPL)
+ Dr. Paul Brey (IPL)
Entomology field collection and identification:
+ Khamsing Vongphayloth (IPL)
+ Sebastien Marcombe (IPL)
+ Khaithong Lakeomany (IPL)
+ Nothasin Phommavan (IPL)
+ Boutsady Somphong (IPL)
+ Somsanith Chonephetsarath (IPL)
+ Phonesavanh Luangamath (IPL)
+ Somphat Nilaxay (IPL)
+ Elliott Miot, PhD student (IPL)
+ Maysa Motoki, Postdoc (IPL)
Insecticide Resistance monitoring:
Sebastien Marcombe (IPL)
Phoutmany Thammavong (IPL)
Somsanith Chonephetsarath (IPL)
Pathogen screening for viruses:
+ Souksakhone Viengphouthong (IPL)
Epidemiology:
+Antony Black (IPL)
+ Virginie Pommelet (IPL)
Background
Vector-borne diseases constitute a significant infectious disease risk for deployed military personnel and for local populations. In Laos, definitive diagnosis is often not available for vector-borne illnesses, so the infectious diseases which are a threat to military and civilian populations are not well-defined. In order to identify common and emerging vector-borne pathogens in Laos, NMRC-A Singapore (SG) has established a study to execute an integrated vector-biology and pathogen discovery platform. The vector samples and related science activities and testing were conducted at the Institut Pasteur du Laos (IPL) laboratory in Vientiane, where a wide range of diagnostic tests can be performed to both identify the vectors and to conduct our training, insecticide resistance epidemiology, and pathogen studies. Aimed at understanding vector-borne threats from several sites within Khammouane, Bokeo, Luang Namtha, Champasak, Borlikhamxay, and Xayaboury provinces, this study is known as the “deployment of an integrated vector-biology and pathogen discovery platform in the Lao PDR” (BIOLAO2). Khammouane, Bokeo, Luang Namtha, Champasak, Borlikhamxay, and Xayaboury provinces are uniquely positioned to serve as study sites and include a large protected area bordering Vietnam in south-central Laos, Thailand in north-west Laos, and both Myanmar and China in the northern Lao regions, where many causes of vector-borne illness are not diagnosed, and from which new infectious diseases may emerge. Vientiane Province has served as a surveillance study site and a diagnostic and training hub for two years on previous vector studies and is an excellent training site for this study. Local staff are already familiar with the requirements of study specimen identification, sample collection, and the principles of vector-biology research.
Strategic objective
+ Collect sandflies, mosquitoes, and associated arthropod specimens from study areas by completion of the study protocol and deliver those samples to the IPL laboratory in Vientiane.
+ Survey and conduct modern ID of indigenous sandfly, mosquito, and tick species’ distribution.
+ Collection, ID, extraction of vector DNA for submission, and development of regional repository. This will provide a valuable resource for downstream modern molecular analysis and genotyping.
+ Perform insecticide resistance tests on Aedes mosquitoes.
+ Establish afeasibility study for a future prospective surveillance study.
+ Identify methodologies, personnel, and programs for the determination of human cohort study field sites, epidemiology, and populations.
+ Building of local capacities and competencies.
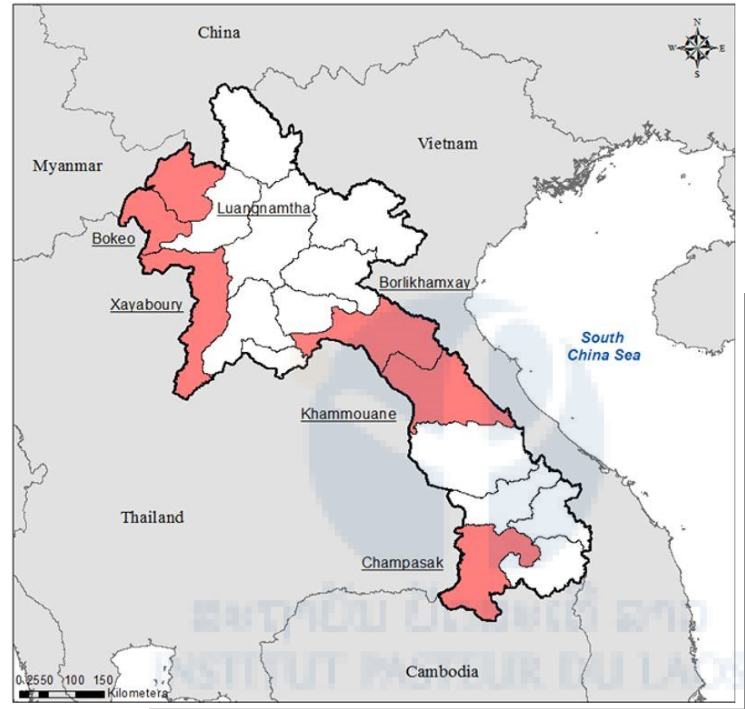
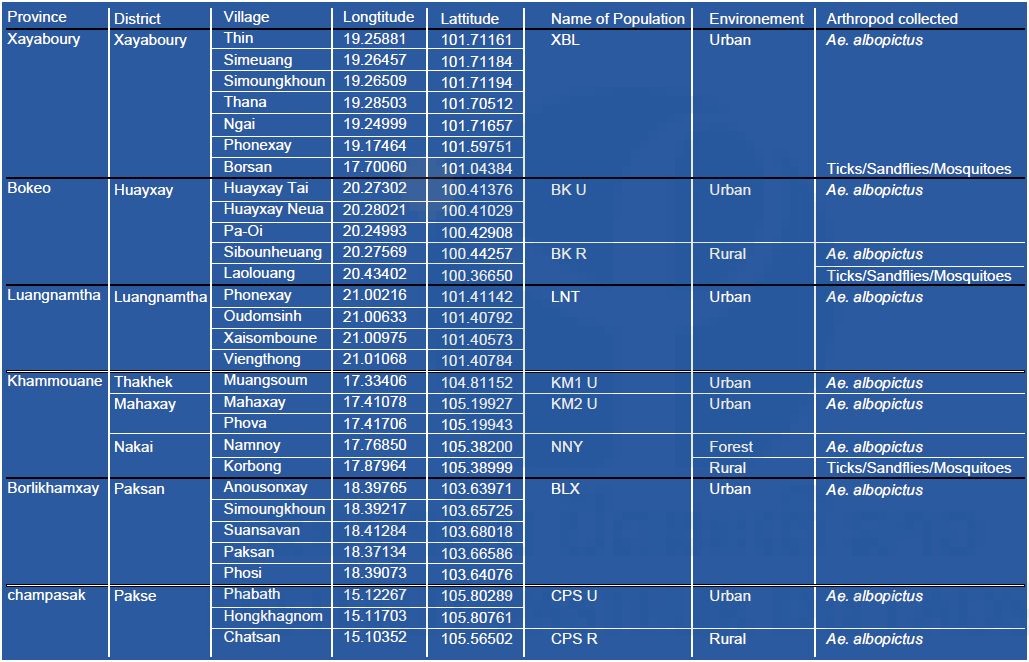
Figure 1: Location of the study sites, Lao PDR, 2017.
Activities
Feasibility study, human cohort study
Introduction
The feasibility study started in Korbong village in January 2017. The aim of this part of the project was to evaluate the possibility of setting up a prospective surveillance study which will involve all IPL labs. This project will focus on vaccine-preventable diseases, circulating and emerging arboviruses, malaria, and Opistorchis viverrini (an endemic liver fluke in the region). These data could be essential for the public health authorities in this area. A questionnaire was prepared in order to lead an effective meeting (cf. report Q1)”
The mission was organized in Khammouane province, Nakai district, Korbong village. The village is located along the Nam Theun River in the Nakai Nam Theun National Protected Area. It takes 3 to 4 hours by boat to access the village from Nakai.

Boat trip on the Nam Theung River
In the village, we had a meeting lasting several hours with the village head, two health center staff, and the representative of the elders. The village head was invested in 2016. One of the IPL entomology staff has formerly worked in the village and established a good relationship with the former village head. The relationship with the new village head seems to have got off to a very good start. We also had a long meeting with the two healthcare workers in the village. We met a representative of the Watershed Management Protection Authority (WMPA) who has a good relationship with IPL and the village authorities. More information can be found at http://www.nt2wmpa.gov.la/en/. In Nakai, we met a representative of the District Health Office (DHO) to discuss the project.
Demographics
The village includes Korbong village and Singthong village, situated a two-hour walk away. Both villages share the same village head, healthcare center, and primary school.
A total of 475 people live in the village: 363 (174 women and 189 men) in Korbong and 112 (48 women and 64 men) in Singthong. The ethnic group is Lao Therng and the religion is Buddhism. The age distribution is presented in Figure 2.
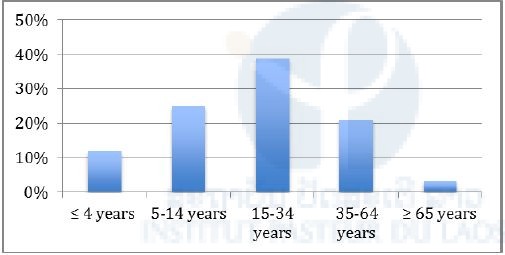
Figure 2: Age distribution in Korbong village, January 2017.
There is one primary school shared between both villages and one primary healthcare center, which was built in 2013.

Primary school
The main occupation of the villagers is farming, mainly rice, corn, and bananas, around the village. There are no latrines in the village, except the two squat toilets in the WNPA office.
Risk factors
Occupational:
Men go into the forest for three or four hours a day to cut wood or to hunt, some stay overnight.
Villagers mainly stay in the village or its surroundings and sometimes go to Nakai by boat. None of them go to Vietnam. Business with Vietnam mainly concerns equipment and doesn’t concern food or medicines.
Water source
Water in the village is mainly provided by a stream uphill from the village and is conducted to the village by a 2000m pipe. Most people boil the water before drinking (when they are at home)
Food
Villagers (mostly men) sometimes eat raw fish and raw meat. They mainly consume local food (meat, fish, rice, vegetables, fruits).
Animals
Livestock (for both villages): 120 buffalo
200 pigs
23 cows
41 goats
2000 chickens During the rainy season, livestock stay under the houses
120 dogs
Wild animals: small monkeys and wild pigs
Arthropod exposure
Villagers mainly complain about tick bites, and mosquitoes in the forest. Villagers notice a lot of mosquitoes around water points. They use bed nets in the village but not when they sleep in the forest. LLINs were handed out in 2009, 2013, and 2015. No insecticides (IRS) are used in the village. Insecticides are used on the crops against crickets and grasshoppers.
Villagers don’t know about Southeast Asian liver fluke and report no malaria cases.
Health situation
Primary healthcare center
The primary healthcare center was built in 2013. Prior to that, village health volunteers provided healthcare. It covers five villages in the area, the furthest one is a 4- to 5-hour walk from Korbong village. (17 53’13” N 105 22’36” E)

The primary healthcare center
Currently, three healthcare workers (two staff and one volunteer) work full time and have been there for six months. They are nurses completing their degrees. A doctor from the district hospital comes every 3–4 months to collect data. In the district, there are two medical doctors and three medical assistants. The dispensary opens every day, with an average of ten patients each day. Consultations are free of charge; patients only pay for the drugs. Healthcare workers can provide medicines for free if necessary. All medical care and drugs are free of charge for children under five years of age. The dispensary is an out-patient department (OPD) but there are two beds for short hospitalizations if needed.
The district supplies the dispensary with medicines and the pharmacy contains basic oral and intravenous (IV) antibiotics, anti-parasitic agents, IV fluids (Ringer’s lactate solution), and vitamins. Medicines are sold to the patients except for children under five years of age. There is no lab available, only a rapid test for malaria.
Outreach clinics are organised every month, mainly for vaccination and primary care.

Pharmacy and vaccines
Health issues in the village
The main complains are fever, gastrointestinal disorders (mainly diarrhea), common cold (‘flu’), and skin conditions. Healthcare workers report no epidemics or major health issues in the six villages. They report no Opistorchis viverrini (endemic liver fluke in the region), malaria, or dengue fever. Nutrition status seems to be assessed by the follow up of weight on weight charts (on the vaccine card). Most of the children are apparently under the curves. Healthcare workers provide general nutritional knowledge to mothers. District officers come once a year for a global nutritional assessment and nutritional awareness promotion. Most babies are born at home with the help of the healthcare workers when possible or a family member. No villagers are trained for deliveries. The healthcare workers give pregnant women basic information on delivery. The health care center doesn’t have any equipment for deliveries whereas this type of facility should have basic equipment such as a delivery table.
Traditional medicine
Healthcare workers report no traditional healers in the villages. People sometimes rely on prayers but herbal medicines or other types of traditional medicine do not seem to be widespread in this area.
Medicines are only provided by the healthcare center, no medicines are sold in the market.
Vaccines
Outreach is organized every month in the five villages. They provide vaccines for new-born babies and follow up the immunization program for the other children. Healthcare workers keep up to date a list of children by name who have been vaccinated and they also give out yellow vaccination cards. Villagers are informed in advance of the outreach day but healthcare workers also go visit the families in their houses. They also provide albendazole and vitamin A every six months.
Vaccines are sent by the district every month and stored in a fridge powered by a solar panel. The fridge was installed one year ago. Prior to that, there was no stock of vaccines in the village and the district provided the vaccines on the day of vaccination outreach. Vaccines were then transported in cool-boxes.
From the healthcare workers’ point of view, acceptance of vaccination is quite good in the villages.
General concerns
Both healthcare workers and the village head are concerned about water and sanitation issues and emphasize the urgent need for decent latrines in the village.
Available data
In the village, healthcare workers collect demographic data along with basic health data: vaccines, numbers of consultations, main complaints, medicines provided, and nutritional assessment (though it is not clear what parameters are being used).
At a district level, data are collected every three months and sent to the different departments:
+ vaccination and health education
+ malaria report system
+ antenatal care
+ mother and child: nutrition.
The district health officer (DHO) is willing to collaborate if data are needed for our project.
Health issues in the district
The representative of the DHO confirmed the information we collected from the healthcare workers.
The main health concerns in the area are diarrhea, upper respiratory tract infections (common cold, ‘flu’) and pneumonia (which is defined as a ‘flu’ that has been on-going for more than three days).
Nutritional assessment is weak and there doesn’t seem to be reliable data.
There are only two or three cases of HIV in the district but there is no systematic screening.
Three cases of suspected leprosy have been reported in a village in the district.

Meeting with the district health officer in Nakai, January 2017
Understanding and acceptance of the scientific project
The village head seems to understand our scientific projects and is willing to collaborate. We were very well welcomed by the village head and villagers seemed to be used to hosting foreigners and were very respectful.
The village head agrees to the entomological part of the project. He has no concerns about stool samples or finger-prick samples. However, he feels reticent about the blood samples and wants to organize a meeting with the villagers to discuss this before giving us his agreement. We explained the aims of the study and the benefits for the population in the future. We also encouraged them to organize meetings and explained we understood their reluctance.
The representative we met at the DHO is willing to help us with this aspect and has offered to organize awareness meetings in the village. He is from the same ethnic group and could be very helpful.
Arthropod collection for pathogen discovery
Methods
Field site collections
In Khammouane province, the first mission was implemented from the 13th to 19th of January to collect arthropods and a second mission for field collection of ticks was carried out between 22th and 25th March, 2017 in both the Korbong village area (Location: 17.879643°N, 105.389993°E), and the Namnoy River area (Location: 17.768548°N, 105.381989°E). A third mission for Aedes mosquito collection was implemented from the 11th to 14th of June in the Namnoy River area.
In the third quarter of the project, two field collections of ticks, mosquitoes, and sandflies were carried out in two provinces.
The first collection was conducted in forest areas in Borsan village located in Borten district, Xayaboury province (location: 17.70060°N, 101.04384°E) from the 3rd to 9th April 2017. The second was conducted in Laolouang village, Houayxay district, Bokeo province (location: 20.43402°N, 100.36650°E) between 29th May and 4th June 2017.

Arthropod collection (ticks and sandflies) in Xayaboury and Bokeo provinces, June 2017, Lao PDR.
Tick collections
A dragging method was used for tick sampling from forest floors and vegetation. These standard dragnets were swept along the forest ground at approximately 1-10 m intervals around natural animal trailways before being examined for ticks. Ticks were removed from the sheets using forceps and transferred to 1.5 ml labeled cryotubes.
All live tick samples were then transported to the Nakai field laboratory. In the laboratory, ticks were placed in the freezer at 20°C for 10 minutes. Then adults, nymphs, and tick larvae were separated, counted, and subsequently transported to IP-Laos in Vientiane Capital and stored at 80°C for further analysis (species identification, pathogen detection, and discovery). All study sites were data-logged for full GPS parameters.
Mosquito and sandfly collection methods
In each selected location, standard collection methods using CDC light traps were used for mosquito and sandfly collections between 4–6 p.m. and 8–9 a.m. All mosquitoes and sandflies were killed by freezing at −20°C for 20–30 minutes. Specimens were stored at −20°C, and then transported to the IP-Laos laboratory in Vientiane Capital for counting and morphological identification. All samples were preserved at −80°C for further pathogen discovery studies.
Identification of ticks, mosquitoes, and sandflies
Ticks were identified and grouped under microscopes in cooling conditions (on ice packs) by using reference determinations from Dr. Richard G. Robbins of the US Armed Forces Pest Management Board (AFPMB), together with related references from Southeast Asia and keys from Japan, Korea, and the Ryukyu Islands (Yamaguti, Tipton, et al. 1972), L. E. Robinson keys for genus Amblyomma (Nuttall, Cooper, et al.) and keys from Thailand (Tanskull and Inlao 1989) for adult Haemaphysalis ticks. As there are no morphological identification keys available for pre-imago forms of Haemaphysalis spp., larval and nymph stages in this genus were grouped by using their main morphological characteristics, especially the capitulum. After tick identification and pooling, all information was registered with the Pathogen Asset Control System (PACS) software and all tick samples were stored at −80°C in IP-Laos, Vientiane Capital, for further analysis.
Mosquitoes were identified under stereomicroscope using related identification keys (Rattanarithikul 2005, Rattanarithikul 2006, Rattanarithikul 2010, Stojanovich and Scott 1965). All samples were separated by species, sex, blood-meal, and site, and then stored at −80°C for further pathogen discovery studies.
For the sandfly specimens, heads, wings, and abdomen genitalia of both sexes were cut under stereomicroscope using sterile needles. The thoraxes were pooled individually, and stored at −80°C for further molecular identification and pathogen discovery studies. Heads, wings, and genitalia were mounted on slides using PVA mediums and morphological identification was carried out under compound microscope using related morphological identification keys and other related references (Lane 1993, Lewis 1978, Lewis 1982, Leng 1987, Léger 2010). As there are no accurate morphological identification keys for sandflies in Laos and surrounding countries, while using the keys above. For sandfly females whose identification was uncertain, they were classified into groups using their morphological characteristics, mainly based on teeth form, wing veins, and spermatheca.
Pathogen discovery protocol
Sample preparation and RNA/DNA extraction
Tick, mosquito, and sandfly samples were pooled into groups of one to ten by species or genus, sex, stage of development, collection period, and site. Specimens were placed in a 1.5 ml vial containing 1 ml of 1X cold Phosphate Buffered Saline (PBS) and Lysing Matrix A zirconium beads (MP Biomedicals; Lysing Matrix E for mosquitoes and sandflies). Sample pools were homogenized for 10 min at a vibration frequency of 25/s in a TissueLyser II system (Qiagen). After grinding, beads and tissues were spun down by centrifugation for 5 min at 3000 rpm. To obtain total nucleic acid (both DNA and RNA) for bacterial and viral detection by polymerase chain reaction (PCR), 100 μl of each pool was extracted and purified by using a NucleoSpin® 8 Virus extraction kit following manufacturer’s protocols. The remaining 400 μl of each pool was stored at –80°C for pathogen isolation.
Arboviral viral screening
Multiple sets of primers have been selected for screening tick specimens for the presence of phlebovirus, flavivirus, and alphavirus sequences; for mosquito specimens for the presence of flavivirus only; and for sandfly specimens for the presence of phlebovirus only by means of conventional nested RT-PCR as previously described (Sanchez-Seco, Rosario et al. 2001; Sanchez-Seco, Rosario et al. 2005).
Bacterial screening
To investigate the occurrence of Rickettsia spp. in ticks in Laos, a molecular screening approach targeting the 17kDa gene was taken (Jiang et al. 2004). The presence of Leptospira spp., Anaplasma phagocytophilum, Ehrlichia chaffeensis, Coxiella burnetii and other Ehrlichia spp., Neorickettsia spp., and Anaplasma spp. was also investigated. The bacterial screening was carried out in collaboration with the Lao-Oxford University-Mahosot Hospital-Wellcome Trust Research Unit (LOMWRU), based at Mahosot Hospital, Vientiane.
Results and achievements
Tick collection and pathogen screening
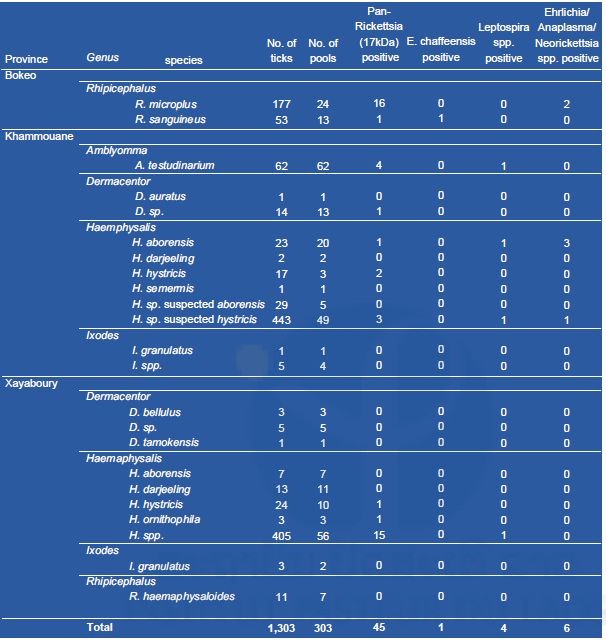
Results of tick collection and pathogen screening are presented in Table1. A total of 1,303 ticks were collected during our field work. Almost all ticks were at the nymphal stage. The most abundant was Haemaphysalis species (967 specimen), followed by Rhipicephalus spp. (241), Amblyomma spp. (62), Dermacentor spp. (24), and Ixodes spp. (9). Rhipicephalus microplus (17) and R. sanguineus (11) were found positive for phleboviruses in Bokeo province. Haemaphysalis hystricis (10) was also found positive for phleboviruses in Khammouane province. In Xayaboury province, one H. darjeeling, one I. granulatus and thirteen Haemaphysalis spp. were found positive for phleboviruses. Only one specimen (H. histricis) from Khammouane was found positive for flaviviruses. None of the ticks tested were positive for alphaviruses.
Table 1. List of the ticks collected in Laos (2017) in three provinces and screened for phlebo, flavi, and alpha-viruses.
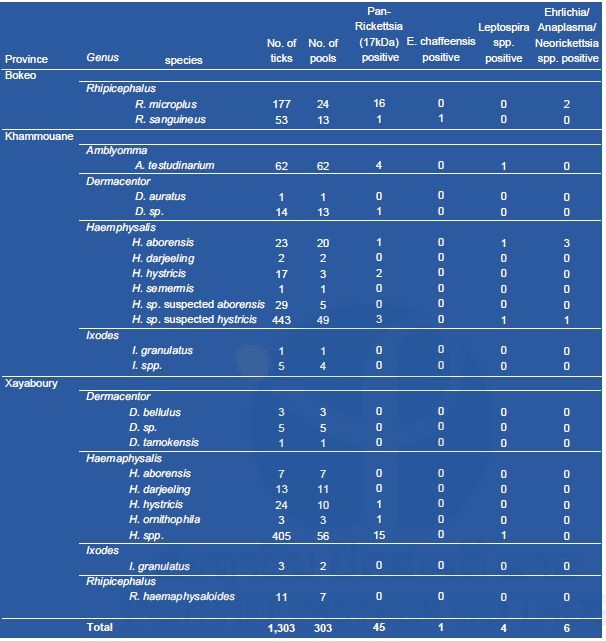
Table 2. List of ticks analyzed for bacteria.
Mosquito collection and pathogen screening
Results of mosquito collection and pathogen screening are presented in the three provinces in 2017 in Table 3. A total of 393 mosquitoes were collected during the course of our survey (Table 3). Only females were collected and among them only forty-four were found blood fed. Fifteen different Anopheles species were identified (n=216). The malaria vector An. minimus was the most represented with 51 specimens followed by An. jeyporiensis with 34 specimens. Ten different Culex species were collected (n=135). Culex tritaeniorhynchus and Cx. quinquefasciatus were the most represented with sixty-two and twenty-one specimens collected respectively. Culex tritaeniorhynchus is a major vector of Japanese encephalitis in Laos. Only one mosquito from Xayaboury was found positive for alphavirus (An. minimus).
Table 3. List of mosquitoes collected in Laos (2017) in three provinces and screened for flaviviruses.
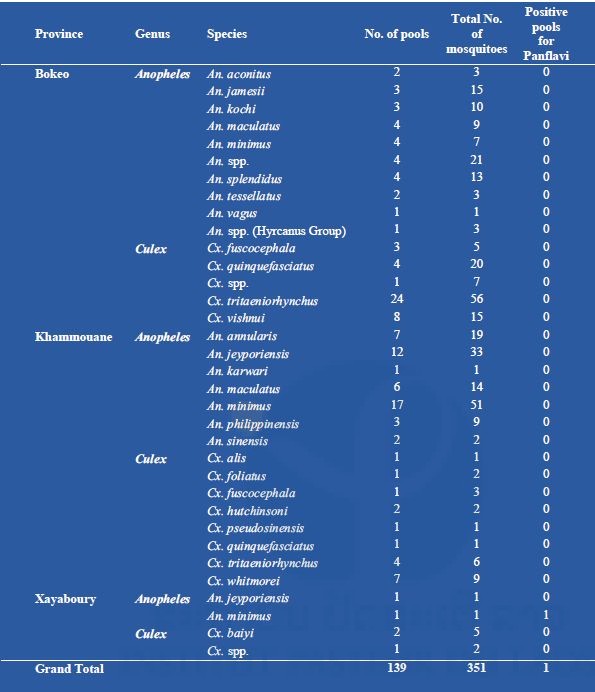
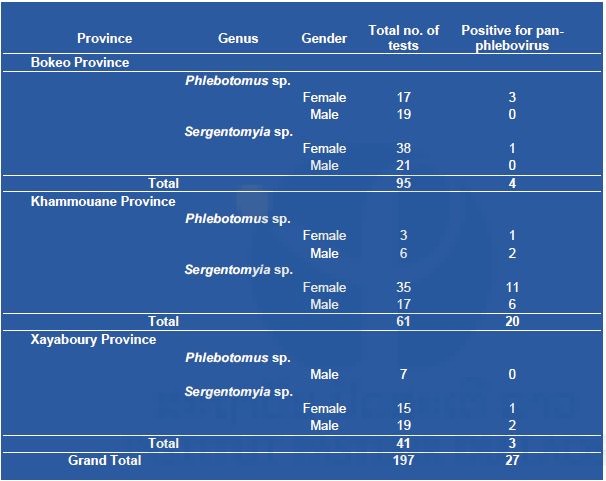
Table 4. List of sandflies screened for phleboviruses in three provinces.
Bionomics of anthropophilic forest mosquitoes from Khammouane Province
Background
Dengue viruses (DENV), Zika (ZIKV), and Yellow Fever (YFV) cause more human morbidity and mortality than any other arthropod-borne viruses (arbovirus). These RNA viruses of the genus Flavivirus originated from sylvatic cycles between non-human primates and forest-dwelling mosquitoes (Holmes & Twiddy 2003). Over the last few centuries, DENV, ZIKV, and YFV have emerged into sustained transmission among humans by Aedes aegypti, a mosquito species with a strong preference for humans. Sylvatic DENV cycles remain active in places such as the jungles of Malaysia and West Africa. Sylvatic ZIKV cycles circulate in the jungles of East Africa. Sylvatic YFV cycles circulate in West Africa and South America. A major concern is the re-emergence of sylvatic DENV strains into the human population (Vasilakis et al. 2011) where they can potentially result in severe disease (Cardosa et al. 2009). Furthermore, recent epidemics of YFV in Angola, DR Congo, Uganda, and most recently Brazil, with several human cases from Africa being introduced into China, have greatly alarmed the public health community (European CDC 2016). Another, less studied concern is the risk of ‘spillback’ of human DENV, ZIKV, and YFV viruses establishing novel sylvatic cycles in Southeast Asia. Spillback has been already documented for yellow fever virus in South America (Hanley et al. 2013). Once entrenched, these putative newly established sylvatic DENV, ZIKV, and YFV cycles would be impossible to eradicate. Therefore, a better understanding of the mosquito fauna in sylvatic environments and especially species with anthropophilic behavior is essential to assess the risk of new emergence/re-emergence.
Methods
Collection site
Mosquito collections were conducted in the forest close to the Nam Noy River in the Annamite range, away from human settlement, in the Nakai Nam Theun 2 Watershed Management and Protection Authority area, Nakai District, Khammouane Province (N17.76850º E105.38200º).
Outdoor Double Bed-Net (DBN) collections
Participants were rested on a long chair inside a small bed-net and were therefore protected from mosquitoes. The small bed-net was covered by a larger one presenting a 30-cm opening at the bottom allowing mosquitoes to enter (Figure 3). The participant served as bait to attract mosquitoes which got stuck in between the two bed-nets. A plastic sheet was placed above the bed-nets to protect the participant from rain.
Every hour, mosquitoes trapped in the space between the bed-nets were collected by the participant using a mouth aspirator and, if necessary, a head torch. Aspirated mosquitoes were carefully transferred into holding cups covered with netting. In the net, a small hole was made to allow the introduction of the aspirator. The hole was plugged with a small cotton wool plug. The cups containing mosquitoes were transferred into a ziplock bag containing an open bottle of chloroform for 30 min in order to kill the mosquitoes. Mosquitoes were then transferred into tubes containing silica gel (5 mosquitoes per tube maximum). Collections were conducted around-the-clock for 6-hour periods at two locations simultaneously by two-person teams for 6 days.
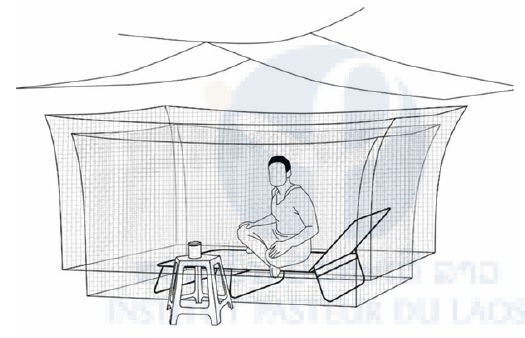
Figure 3: Double bed-net system.
Mosquito identification
Mosquitoes were pinned and identified morphologically following Rattanarithikul et al. (2005, 2006, 2007 & 2010) keys.
Ethics statements
This study was approved by the Lao Ministry of Health (6 April 2017, nº190). All participants signed an informed consent form before participating in the study. They were supervised during the full length of the mission by members of the Institut Pasteur du Laos.
Results
Out of the 355 female mosquitoes captured, 24 different species from 9 genera were identified (Table 5). Seven species had never been recorded in Laos before this study.
Table 5. Species collected in the double bed-net with number of individuals (N).
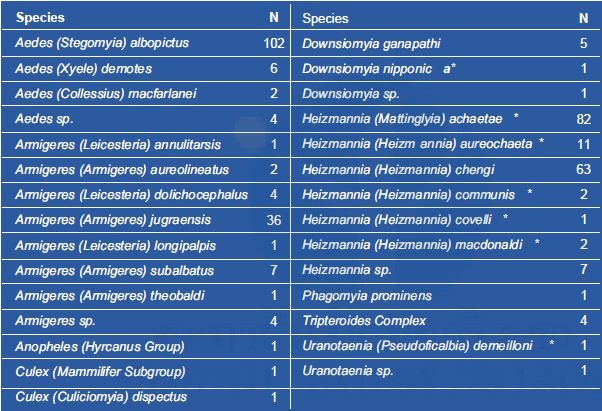
* indicates a new record in Laos
Three genera were predominant: Heizmannia (47%), Aedes (32%), and Armigeres (16%) (Figure 4). Heizmannia and Armigeres genera had a higher species diversity (i.e. 7 & 6 different species) compared to Aedes (i.e. 3 species, with Ae. albopictus largely predominant).
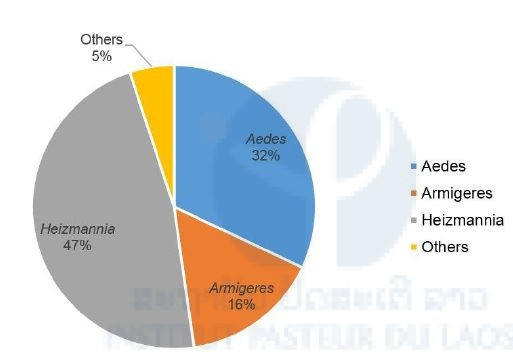
Figure 4: Proportion of mosquitoes for each genus.
Relative abundance analysis showed that four species are more represented in our captures: Ae. albopictus, Hz. achaetae, Hz. Chengi, and Arm. jugraensis with 28.7%, 23%, 17.7%, and 10.1% respectively (Figure 5).
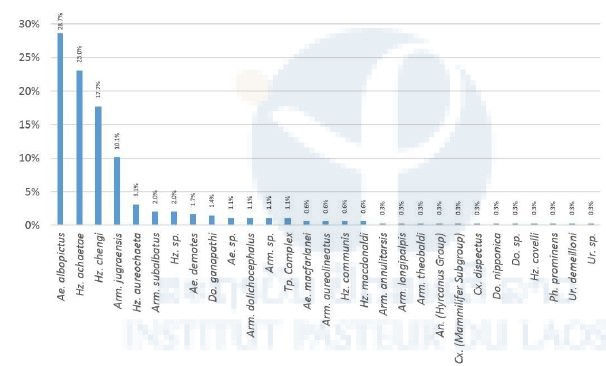
Figure 5: Relative abundance for each mosquito species.
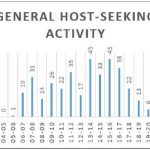
Host-seeking analysis showed that anthropophilic mosquitoes in this area were diurnal, with almost no captures from dusk (around 19:00) until dawn (around 06:00) (Figure 6A). Two peaks of activity could be observed, one in the morning right after sunrise and a bigger one in the afternoon from 13:00 to 18:00 (Figure 6A). The same analysis was done on the three most represented species:
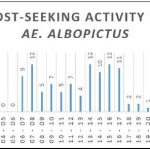
+ Ae. albopictus was active all day but particularly during two periods: right after sunrise and then in the afternoon from 13:00 to 17:00 (Figure 6B).
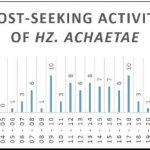
+ Hz. achaetae was active all day long from 06:00 to 19:00 with no distinct peak of activity (Figure 6C).
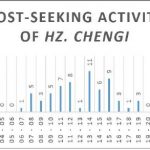
+ Hz. chengi gradually became active from 06:00 to 12:00 and from 14:00 its activity began to decrease gradually until 19:00 (Figure 6D).
Conclusion
Our study showed that at least 24 species of sylvatic mosquitoes present an opportunistic anthropophilic behavior previously undescribed for most of them. This biting behavior could be the link between wildlife and humans in disease transmission. For example, Ae. albopictus is a well-known vector of arboviruses such as dengue virus, chikungunya virus, or zika virus. A better insight (i.e. host-seeking activity, abundance, etc.) on those potential bridge vectors is therefore of vital important to prevent the emergence of new pathogens.
Figure 6: Host-seeking activity. Number of mosquitoes captured per time slot for all mosquitoes and for the three most represented species.
Insecticide resistance of Aedes albopictus populations
Methods
Field site collection, sampling procedure and mosquito identification
During the rainy season of 2017 (May to July), collection of Aedes mosquitoes were carried out in 29 villages in six different provinces (Xayaboury, Bokeo, Luang Namtha, Khammouane, Champasak, and Borlikhamxay provinces, Figure 1). All the collection sites were geo-referenced (Figure 1). Collections were made by the IPL staff in collaboration with the district and provincial health officers in each location selected for the project. Larvae were collected in breeding habitats from households and temples in the twenty-three villages and brought back to the laboratory at IP-Laos for rearing. After adult identification, mosquitoes obtained were separated by species and locations. Ae. albopictus were kept for breeding. Female mosquitoes were then blood fed using quail, and the eggs obtained were kept for the larval and adult bioassays. Nine Ae. albopictus colonies have been established in IP-Laos for insecticide resistance studies.

Susceptibility bioassays methods
Larval bioassays were carried out using technical grades of temephos at the recommended diagnostic dose (0.0132 mg/L), deltamethrin (0.00132 mg/L), permethrin (0.014 mg/L), and DDT (0.04 mg/L) according to WHO guidelines. Bioassays were performed using late third- and early fourth-instar larvae of the field strains. For each bioassay, larvae of each strain were transferred to cups containing 99 mL of distilled water and 1 mL of the insecticide tested at the desired concentration. Larval mortality was recorded after an exposure of 24 hours. Following WHO criteria, a population is considered resistant if the mortality after 24 hours is under 90%, resistance is suspected with mortality between 90 and 98%, and a population is susceptible with mortality over 98%.
Adult bioassays were carried out using filter papers treated with diagnostic doses of deltamethrin (0.05%), permethrin (0.25%), DDT (4%), and malathion (0.8%) following a WHO protocol (Table 4). Mortality resulting from tarsal contact with treated filter papers was measured using WHO test kits on adult mosquitoes. Four batches of 25 non-blood-fed females (2–5 days of age) were introduced into holding tubes and maintained for 60 minutes at 27 ± 2°C and a relative humidity of 80 ± 10%. Insects were then transferred into the exposure tubes and placed vertically for 60 minutes under subdued light. Mortality was recorded 24 hours after exposure. Following WHO criteria, a population is considered resistant if the mortality after 24 hours is under 90%, resistance is suspected with mortality between 90 and 98%, and a population is susceptible with mortality over 98%.
Results
The results of the larval bioassays are presented in Table 6. All larval Ae. albopictus populations tested were highly resistant to temephos and DDT. Temephos mortality ranged from 29% to 73%, DDT mortality from 5% to 37%. The two populations of Xayaboury and Bokeo showed a reduced susceptibility to deltamethrin while all the other populations tested were resistant. Surprisingly, all the populations were susceptible to the other pyrethroid, permethrin.
Table 6. Resistance status of Aedes albopictus (Larvae) to temephos, deltamethrin, permethrin, and DDT.
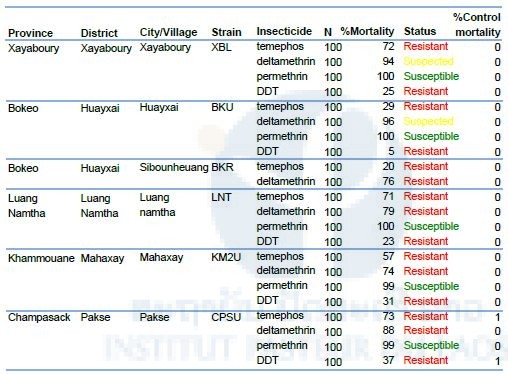
Diagnostic doses of temephos, deltamethrin, permethrin, and DDT are 0.0132, 0.00132, 0.014 and 0.04 mg/L.
The results of the adult bioassays are presented in Table 7. All the population tested against deltamethrin were susceptible except the population from Pakse in Champasak province (91% Again, all the populations tested against the other pyrethroid, mortality). permethrin, were susceptible except in the two populations from Champasak with mortalities of 97% and 98%. All the populations were resistant to malathion (mortality<90%) except in Luang Namtha where resistance is suspected (90.4% mortality). Finally, all the populations were resistant or suspected resistant to DDT with mortality ranging from 22% to 91%.
Table 7. Resistance status of Aedes albopictus (adult) to deltamethrin, permethrin malathion, and DDT.
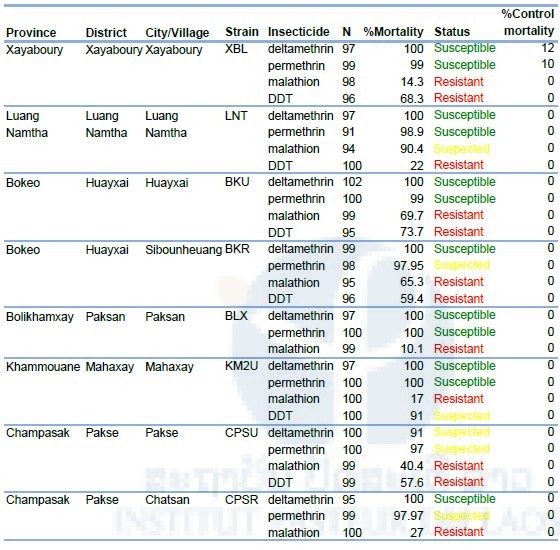
Diagnostic doses of deltamethrin (0.05%), permethrin (0.25% ), DDT (4%), and malathion (0.8%) were used.
Analysis of genetic differentiation of Aedes albopictus population from Laos
Background
The aim of this study is to investigate the population genetic structure of Ae. albopictus in eight provinces in the Lao PDR using a COI mitochondrial DNA marker. The genetic diversity and population structure are important to understand the evolution of this species and would be essential for implementation of dengue control programs, as well as to evaluate the insecticide resistance spread.
Material and Methods
Adults or larvae of Aedes albopictus were collected in eight provinces in the Lao PDR (Figure 7; Table 8. The collections were carried out in the following areas of the Lao PDR: north-west (Bokeo, Luang Namtha, and Xayabury provinces), north (Luang Prabang province), central (Vientiane prefecture, Borikhamxay and Khammouane provinces) and south (Champasak province). Specimens were all morphologically verified as Ae. albopictus using the available keys (Rattanarithikul et al. 2010).
Table 8. Collection information for Aedes albopictus.
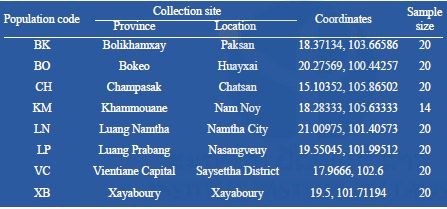
Figure 7: Sampling locations of Aedes albopictus in the Lao PDR.
DNA extraction and sequencing
Total genomic DNA was extracted from a single whole mosquito according to manufacturer’s instructions (Macherey-Nagel). The fragment of mtDNA COI gene was amplified using the two sets of primers 1454F (5’ GGTCAACAAATCATAAAGATATTGG 3’) and 2160R (5’TAAACTTCTGGATGACCAAAAAATCA3’); 2027F (5’CCCGTATTAGCCGGAGCTAT 3’) and 2886R (5’ATGGGGAAAGAAGGAGTTCG3’) (Zhong et al. 2013). The polymerase chain reaction (PCR) protocol utilized is detailed explicitly in Zhong et al. (2013). Products of PCR were visualized on 1.5% agarose gels and then purified using ExosapIT® (USB Co, Cleve¬land, OH, USA). All sequencing reactions were carried out in both directions using ABI Big Dye Terminator Kit v.3.1 (PE Applied Biosystems, Warrington, England), analyzed on an ABI Prism 3500xL – Avant Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Data analyses
The COI gene sequences from 140 mosquitoes were edited using Sequencher® version 5.4.6 (Gene Codes Corporation, Ann Arbor, USA) and automatically aligned in CLUSTAL X (Thompson et al. 1997). The number of haplotypes (H), haplotype diversity (Hd), and nucleotide diversity (π) within each site were generated using DnaSP version 5.0 (Librado and Rozas 2009).
Phylogenetic analyses was conducted by the neighbor-joining (NJ) method in MEGA v. 7 (Kumar et al. 2016). Genetic differentiation among populations (FST ), gene flow (Nm), Tajima’s D value (Tajima 1989), and Fu’s FS statistics (1997) were estimated using Arlequin v. 3.5 (Excoffier et al. 2005). A mismatch distribution was performed to distinguish between a smooth unimodal distribution and a multimodal, or ragged, distribution (Slatkin and Hudson 1991; Rogers and Harpending 1992; Rogers 1995).
Results
Partial sequences of the mtDNA COI (1337-bp) were amplified from 155 specimens, representing 8 populations of Ae. albopictus. No insertion, deletion, or stop codon was detected across all samples. The conserved sites were 1301, variables sites 36. Of these, 16 were singleton and 20 parsimonious informative. A total of 46 haplotypes were identified; of these, 23 haplotypes (50%) were shared and 23 (50%) were singletons (Table 9). The most common haplotypes were HAP2 (n = 42) and HAP13 (n = 18). HAP2 shared among all populations; HAP13 shared among 3 populations, Bokeo (BO), Luang Namtha (LN) and Xayaboury (XB) (Table 9). Phylogenetic analysis showed the 46 haplotypes identified in one clade with no genealogical pattern (Figure 8). The average number of nucleotide differences ranged from 0.537 (LN) to 3.105 (KM), corresponding with the range of the nucleotide diversity (π) 0.0040 (LN) – 0.0023 (VC). The range of haplotypes diversity (Hd) was from 0.416±0.116 (mean ± standard deviation, SD) (LN) to 0.942±0.029 (VC) (Table 13). The fixation index FST of Ae. albopictus COI found the highest level of genetic differentiation between LN and XB (FST = 0.37135; p<0.05). The only negative value was in BK/CH, which indicated very limited genetic differentiation between these populations. Gene flow (Nm) was below 1 (0.874646) between LN and XB, but > 1 among remaining populations (Table 11).
Table 9. Haplotypes of Aedes albopictus from the Lao PDR based on the mtDNA COI marker.
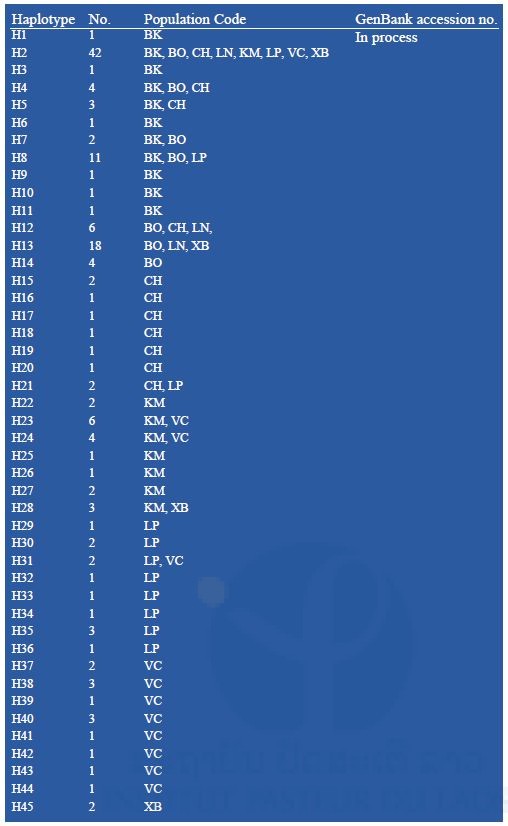
Table 10. Summary of haplotype and nucleotide diversity measures of the COI gene for Ae. albopictus from Laos. N = number of individuals analyzed; H = number of haplotypes, Hd = haplotype diversity; SD = standard deviation; π = nucleotide diversity; K = average of nucleotide differences.
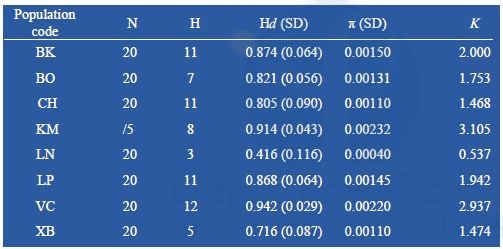
Table 11. Pairwise differentiation (FST, below the diagonal), and gene flow (Nm, above diagonal) among populations of Ae. albopictus from the Lao PDR.
*statistically significant, p<0.05
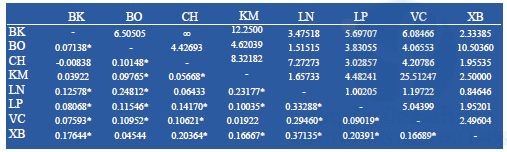
Figure 8: Phylogenetic relationship among 46 haplotypes of Ae. albopictus from the Lao PDR inferred by neighbor-joining method. Ae. aegypti was employed as outgroup.
Conclusion
Population genetics could provide a better understanding of the degree of population spread and establishment of Ae. albopictus, facilitating dengue prevention and vector control. In this study, Ae. albopictus populations showed low genetic differentiation and high gene flow among populations. Absence of the genetic structure among populations indicated a recent rapid range expansion evidenced by the high haplotype diversity contrasting with the low nucleotide diversity.
In future studies, more samples should be involved from other provinces in the Lao PDR and more genetic markers could be verified.
Vector-borne Disease and Control Training
Training sessions were organized for public health officers from provinces and districts where insecticide resistance was monitored (Xayaboury, Bokeo, Luang Namtha, Khammouane, Champasak and Borlikhamxay provinces). For each province and district, two to three officers were trained on mosquito control, insecticide resistance, and mosquito collection. A training session was held by Dr. Marcombe at the Institut de la Francophonie pour la Médecine tropicale in November 2016 to the Master class of 2016–2017. The topic of the module taught was on Public Health, specifically dengue mosquito control and insecticide resistance in Laos with the specific example of the Biolao2 Project.
Conclusion
Pathogen discovery platform
Virology screening
Ticks and sandflies are well known vectors of numbers of human and animal pathogens worldwide. However, their distribution and their putative role in human and animal health remain to be determined in a number of countries, including the Lao PDR.
Arbovirus screening performed in the course of the Biolao2 Project confirmed the high level of positivity of ticks and sandflies for arboviral sequences previously observed by our teams. At this stage, it is not possible to draw a link between the presence of the viral sequences and human and/or animal infections in the Lao PDR but some arguments are supporting a potential threat for humans and domestic animal species: (i) the high level and the diversity of tick and sandfly species and (ii) the detection of positive male specimens suggests a stable maintenance of the virus in sandfly populations through vertical and/or horizontal transmission.From a virologic point of view, it is now of main concern to further document the viral pathogens detected. Specific strategies for preliminary sequencing will be applied to pre-select positive specimens for deep sequencing. In parallel, the remaining biological material will be used for viral isolation assays with the aim of producing viral antigens to develop serological tools that may help screening human populations living in close contact with the putative vectors detected during this project.
Bacteriology screening
Rickettsia spp. was identified in 45/303 (14%) of all tick pools, a high percentage compared to our previous observation (5%) (Taylor et al. 2016). Six of the 303 pools were positive for the Ehrlichia/Anaplasma/Neorickettsia pan-PCR and further analysis and sequencing identified Anaplasma bovis, Anaplasma marginal, and Ehrlichia canis. Interestingly, 4/303 tick pools were positive for Leptospira spp. This is the first data available on Leptospira spp. in Laotian ticks; further investigations into their role as causes of human disease and of ticks as their vectors are needed.
Insecticide resistance
Vector control is currently the most effective way to combat dengue, chikungunya, and Zika. However, the identification of proper control methods has been challenging due to the variable bionomics of Ae. albopictus and limited knowledge about its resistance status. This study has highlighted the importance of expanding Ae. albopictus control in urban and rural villages.
Temephos insecticide is the active ingredient of the Abate formulation, the larvicide used for dengue vector control in the Lao PDR. Those levels of insecticide resistance indicate that a selection pressure has occurred in these mosquito populations. This emphasizes the need to find a substitute for the Abate formulation to avoid the development of resistance, thus rendering future public health operations useless. Furthermore, Abate is posing environmental issues and was banned in the European Union in 2009. The future use of more environmentally friendly products such as Bti, pyriproxyfen, and diflubenzon is recommended, especially knowing that no cross-resistance between these insecticides and temephos has been reported.
Better understanding of the levels and mechanisms of insecticide resistance will help to improve the surveillance, the management, and the control of insecticide-resistant References dengue vectors in the Lao PDR. The development of new tools such as molecular biomarkers (i.e. gene Copy Number Variations [CNVs], Single Nucleotide Polymorphisms [SNPs] and differentially expressed genes [DE genes]; Faucon et al. 2015) is urgently needed to fight against dengue and to contribute to a fastest, costless and more efficacious control operations in resistant-mosquito population areas.
RECOMMENDATIONS for Aedes albopictus CONTROL:
+ It is important to maintain a regular monitoring of the levels of insecticide resistance in the different provinces of the Lao PDR to detect the development of insecticide resistance in the dengue vectors
+ We recommend the use of alternative insecticides where insecticide resistance is detected+ We recommend to start using new insecticides in rotation (i.e. a different insecticide each year) to avoid or to manage the development of insecticide resistance in the larval and adult populations of the dengue vectors in the Lao PDR
+We encourage the use of biological control such as larvivorous fish species or the copepod Mesocyclops in large water bodies
Publications in progress
Marcombe S, Chonephetsarath S, Thammavong P, Somphong B, Phommavan N, Hertz JC, and Brey PT. Insecticide resistance status of Aedes albopictus populations in the Lao PDR.
Motoki MT, Miot E, Thammavong P, Chonephesarath S, Phommavanh N, Hertz JC, Marcombe S, and Brey PT. Genetic population of the Asian tiger mosquito Aedes albopictus (Diptera: Culicidae) in the Lao PDR inferred by mitochondrial COI gene.
References
Cardosa J, Ooi MH, Tio PH, Perera D, Holmes EC, Bibi K, Abdul Manap Z (2009). Dengue virus serotype 2 from a sylvatic lineage isolated from a patient with dengue hemorrhagic fever. PLoS Negl Trop Dis 3: e423.
European Centre for Disease Prevention and Control (ECDC). Rapid Risk Assessment. Outbreak of yellow fever in Angola, Democratic Republic of Congo and Uganda: First update, 27 May 2016. Stockholm: ECDC; 2016. [Accessed: 30/10/2017] http://ecdc.europa.eu/en/publications/Publications/RRA-Yellow%20fever-first-update-Angola-China-DRC-Uganda-May-2016.pdf
Excoffier L, Laval G, Schneider S (2005). Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol Bioinf Online 1:47-50.
Faucon F, Gaude T, Dusfour I, Navratil V, Corbel V, Juntarajumnong W, Girod R, Poupardin R, Boyer F, Reynaud S, and David J-P (2017). In the hunt for genomic markers of metabolic resistance to pyrethroids in the mosquito Aedes aegypti: An integrated next-generation sequencing approach. PLoS Negl Trop Dis 11: e0005526.
Faucon F, Dusfour I, Gaude T, Navratil V, Boyer F, Chandre F, Sirisopa P, Thanispong K, Juntarajumnong W, Poupardin R, Chareonviriyaphap T, Girod R, Corbel V, Reynaud S, David JP (2015). Unravelling genomic changes associated with insecticide resistance in the dengue mosquito Aedes aegypti by deep targeted sequencing. Genome Res, 2015 Jul 23.
Grigoraki L, Balabanidou V, Meristoudis C, Miridakis A, Ranson H, Swevers L, and Vontas J (2016). Functional and immunohistochemical characterization of CCEae3a, a carboxylesterase associated with temephos resistance in the major arbovirus vectors Aedes aegypti and Ae. albopictus. Insect Biochem and Mol Biol 74: 61–67.
Grigoraki L, Lagnel J, Kioulos I, Kampouraki A, Morou E, Labbé P, Weill M, and Vontas J (2015). Transcriptome Profiling and Genetic Study Reveal Amplified Carboxylesterase Genes Implicated in Temephos Resistance, in the Asian Tiger Mosquito Aedes albopictus. PLoS Negl Trop Dis 9: 1–17.
anley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, and Vasilakis N (2013). Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol 19: 292–311.
olmes EC and Twiddy SS (2003). The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol 3: 19–28.
Kasai S, Komagata O, Itokawa K, Shono T, Ng LC, Kobayashi M, and Tomita T (2014). Mechanisms of Pyrethroid Resistance in the Dengue Mosquito Vector, Aedes aegypti: Target Site Insensitivity, Penetration, and Metabolism. PLoS Negl Trop Dis 8.
Kent RJ (2009). Molecular methods for arthropod bloodmeal identification and applications to ecological and vector-borne disease studies. Mol Ecol Resour 9: 4–18.
Kumar S, Stecher G, and Tamura K (2016). MEGA7. Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874.
Librado P and Rozas J (2009). DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452.
Leger N, Depaquit J, and Gay F (2010). Chinius eunicegalatiae n. sp. (Diptera; Psychodidae), a cavernicolous sandfly from Laos. Ann Trop Med Parasitol 104(7): 595–600.
Leng YJ (1987). A preliminary survey of phlebotomine sandflies in limestone caves of Sichuan and Guizhou Provinces, south-west China, and description and discussion of a primitive new genus Chinius. Ann Trop Med Parasitol 81(3): 311–317.
Lewis, DJ (1978). The phlebotomine sandflies Diptera: Psychodidae of the Oriental Region. Bull Br Mus Nat Hist Entomol 37(6): 217–343
Lewis, DJ (1982). A Taxonomic Review of the Genus Phlebotomus (Diptera, Psychodidae). Bull Br Mus Nat Hist Entomol 45(2): 121–209.
Nuttall GHF, Cooper WF, Warburton C, Robinson LE, and Arthur DR (1926). Ticks: pt. IV. The genus Amblyomma. Cambridge University Press.
Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Jones JW, and Coleman RE (2005). Illustrated keys to the mosquitoes of Thailand. II. Genera Culex and Lutzia. Southeast Asian J Trop Med Public Health 36 Suppl 2: 1–97.
Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P, Jones JW, and Coleman RE (2006). Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian J Trop Med Public Health 37 Suppl 2: 1–128.
Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Coleman RE, and Richardson JH (2010). Illustrated keys to the mosquitoes of Thailand. VI. Tribe Aedini. Southeast Asian J Trop Med Public Health 41 Suppl 1: 1–225.
Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Jones JW, and Coleman RE (2007). Illustrated keys to the mosquitoes of Thailand V. Genera Orthopodomyia, Kimia, Malaya, Topomyia, Tripteroides, and Toxorhynchites. Southeast Asian J Trop Med Public Health 38 Suppl 2: 1–65.
Rogers AR (1995). Genetic evidence for a Pleistocene population explosion. Evolution 49: 608–615.
Rogers AR and Harpending H (1992). Population growth makes waves in the distribution of pairwise differences. Mol Biol Evol 9: 552–569.
Slatkin M and Hudson RR (1991). Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129: 555–562.
Stojanovich CJ and Scott HG (1965). Illustrated Key to Aedes Mosquitoes of Vietnam. U.S. Department of Health, Education and Welfare, Public Health Service, Communicable Disease Center.
Tanskull P and Inlao I (1989). Keys to the adult ticks of Haemaphysalis Koch, 1844, in Thailand with notes on changes in taxonomy (Acari: Ixodoidea: Ixodidae). J Med Entomol 26(6): 573–600.
Tajima F (1989). Statistical method for testing the neutral mutation hypothesis by DNA polymorphisms. Genetics 123: 585–595.
Thompson J, Gibson T, Plewniak F, Jeanmougin F, and Higgins DG (1997). The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Ac Rs 25: 4876–4882.
Vasilakis N, Cardosa J, Hanley KA, Holmes EC, Weaver SC (2011). Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol 9: 532–541.
Yamaguti N, Tipton VJ, Keegan HL, and Toshioka S (1971). Ticks of Japan, Korea, and the Ryukyu Islands. Brigham Young Univ sci bull Biol ser 15(1):1.
Zhong D, Lo E, Hu R, Metzger ME, Cummings R, Bonizzoni M, Fujioka KK Sorvillo TE, Kluh S, Healy SP, Fredregill C, Kramer VL, Chen X, and Yan G (2013). Genetic analysis of invasive Aedes albopictus populations in Los angeles County, California and its potential public health impact. PLos One. 8(7): e68586.