Surveillance of arboviruses in Lao PDR
 Collaboration
Collaboration
• 10 military hospitals from 8 provinces in Lao PDR.
• National Centre for Laboratory and Epidemiology (NCLE), Ministry of Health, Lao PDR.
• Department of Communicable Diseases Control (DCDC), Ministry of Health, Lao PDR.
Funding
• Defense Threat Reduction Agency (DTRA), USA.
• French Ministry of Higher Education, Research and Innovation (MESRI), France, through funds allocated by Institut Pasteur de Paris.
• World Health Organization, Lao PDR country office.
Objectives
The main objective of the arbovirus surveillance program implemented at the IPL since 2012 is to provide the Lao Ministry of Health (MOH) with critical information regarding the circulation of various arboviruses, particularly DENV and CHIKV, within the country. This data supports the MOH in monitoring and controlling the spread of these viruses to protect public health.
Background
Since 2012, the Virology laboratory has been providing the Lao MOH with information on the circulation of arboviruses within Lao PDR. To achieve this goal, the Virology laboratory has established a surveillance network for arboviruses in the Vientiane capital. In 2015, at the request of the MOH, the surveillance was extended to the 2 southern provinces of Laos (Saravane and Attapeu). Since 2018, it has been extended to an additional 7 provinces (Champasack, Savannakhet, Luangprabang, Xiengkhuang, Phongsaly, Oudomxay and Vientiane province) through the implementation of the Arboshield project, a collaboration with the Lao Army Health Service.
Methodology
Samples.
Patient blood samples are collected on EDTA tubes and stored at +4°C prior to transportation to IPL for diagnosis and molecular characterization. At IPL, blood specimens are centrifuged and plasma samples are used to perform the various virological tests.
Virus, antigen and antibodies detection.
Virus nucleic acids are extracted from human plasma by NucleOspin RNA virus kit (Macherey-Nagel); NucleOspin 8 virus kit (Macherey-Nagel) or Nucleic Acid Extraction Rapid kit (Bioperfectus) according to the manufacturer’s instructions.
Extracted RNAs are then screened for presence of dengue (DENV) and Zika (ZIKV) viruses by a duplex onestep real-time RT-PCR assay (1). The DENV serotype identification is performed by using a multiplex onestep real-time RT-PCR assay (1). The plasma samples that test negative for DENV by PCR are screened by the Dengue NS1 Antigen + Antibodies Combo rapid test (SD Bioline) for detection of DENV NS1, anti-DENV IgM and anti-DENV IgG. Finally, the samples that test negative for DENV by PCR and/or DENV NS1 by rapid test are then screened for the detection CHIKV using a real-time RT-PCR assay (2).
Results
Dengue surveillance.
Between 1st of January and 11th of October 2024, a total of 195 suspected cases were investigated at IPL. The samples were nearly evenly distributed between hospitals in Vientiane Capital (44.9%, n=88) and provincial hospitals (55.1%, n=107), and patients originated from 12 provinces (Figure 1).
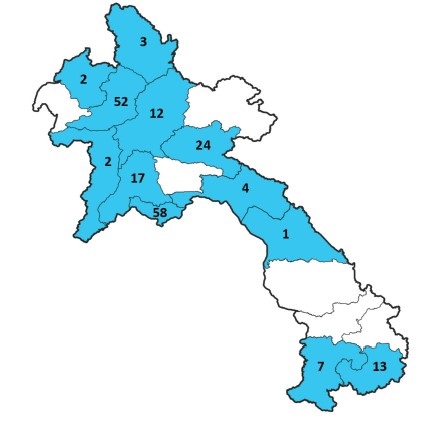
Figure 1: Origin of the samples collected for the arbovirus surveillance, received at IPL between 01 January and 11 October 2024.
Of the 195 samples analysed, 26.5% were classified as “confirmed acute dengue infection cases” (positive by RT-PCR or dengue NS1 test), while 8.2% were identified as “acute or recent dengue infection” (presence of anti-dengue IgM). Another 14.8% were classified as “inconclusive” (only anti-dengue IgG detected), and in 49.5% of the cases, no evidence of dengue virus infection was found. One sample was deemed non-compliant due to insufficient volume for full analysis.
Serotype identification.
Between 1st January and 11th October 2024, dengue serotyping was successfully performed on all 52 samples positive by DENV PCR. At the national level, DENV serotype 2 (DENV-2) was the predominant serotype in the country representing 86.5% of all PCR-positive cases and the DENV serotype 1 (DENV-1) was detected in 13.5% of cases.
Differential diagnosis.
The algorithm used to investigate dengue suspected cases at IPL includes a differential diagnostic approach. Since 2023, all samples received at IPL were screened by a duplex RT-PCR to detect DENV and ZIKV. Subsequently, all samples that tested negative by DENV/ZIKV PCR were then tested for CHIKV infection by RT-PCR. One acute CHIKV infection was detected (CHIKV PCR positive) in one sample from Vientiane Province but no ZIKV was detected among the samples screened in 2024. DENV subtyping, if needed, is available at IPL on request from partners.
Discussion.
Until week 41 of 2024, Laos reported 16,966 suspected cases, including 11 fatal cases. During this period, IPL tested blood specimens from 195 patients with suspected dengue fever. Among them, 52 (26.5%) cases were classified as “confirmed acute dengue infection”, 16 (8.2%) as “acute or recent dengue infection”, 29 (14.8%) as “inconclusive” and 98 (49.5%) showed no evidence of dengue infection.
The number of samples tested this year significantly decreased compared to previous years due to a reorganization of IPL’s dengue surveillance activities.
In 2024, DENV-2 was the predominant serotype detected among DENV PCR-positive samples, accounting for 86.5% of cases. This confirms the shift in serotype distribution observed in 2023, when DENV-2 emerged as the dominant serotype. In contrast, DENV-1 was the most frequently detected serotype in 2021 and 2022, CHIKV is mostly circulating in the southern provinces of Lao PDR (3). Due to limited diagnostic capacity in local hospitals and the fact that some physicians may not be fully familiar with the complete spectrum of chikungunya symptoms, which can sometimes resemble those of dengue, passive surveillance of CHIKV is crucial for tracking its circulation. ZIKV has also been identified as a potential threat in Lao PDR, having already been detected in neighboring countries such as Cambodia, Thailand, and Vietnam (4). In February 2020, Lao PDR reported its first probable case of congenital Zika, when a baby was born with microcephaly in Vientiane. Medical, epidemiological, and laboratory investigations confirmed the case (5). Given the overlapping symptoms of these arboviruses, the diagnostic algorithm used at IPL to investigate suspected dengue cases includes CHIKV and ZIKV in the differential diagnosis. Since 2023, all samples received at IPL have been screened using a duplex RT-PCR, which detects both DENV and ZIKV simultaneously (1). Additionally, all samples that test negative for DENV by PCR are further screened for CHIKV infection using RT-PCR (2). In 2024, one acute CHIKV infection was detected in one patient coming from Vientiane Province, with no history of travel inside or outside the country. This finding is interesting as until now, the majority of CHIKV cases were detected in southern provinces and the few cases detected in Vientiane Capital were imported cases (3).
Conclusion and perspectives.
In 2024, 52 samples (26.5%) were classified as “confirmed acute dengue infection,” and 16 samples (8.2%) as “acute or recent dengue infection” out of 195 samples collected from patients with dengue-like symptoms at hospitals participating in the surveillance system. One acute CHIKV infection was detected in a patient from Vientiane province, while no ZIKV infections were detected.
The results of the arbovirus surveillance were shared through multiple channels: i) daily updates were provided to clinicians, ii) weekly reports were sent to NCLE and DCDC, and iii) monthly summaries were shared with DCDC. Both NCLE and DCDC consolidate testing data from various laboratories and report aggregated information to the Ministry of Health (MOH). This active surveillance enables the MOH to monitor the epidemiologic situation of dengue in Laos effectively.
In the future, advanced sequencing methods, such as next-generation sequencing (NGS), could also enable full-length genome characterization of DENV and CHIKV, which would enhance our understanding of the dynamics of these viruses’ circulation in Lao PDR.
References
1. Ou TP, Yun C, Auerswald H, In S, Leang R, Huy R, et al. Improved detection of dengue and Zika viruses using multiplex RT-qPCR assays. Journal of Virological Methods. 2020 Aug;282:113862. 2. Pastorino B, Bessaud M, Grandadam M, Murri S, Tolou HJ, Peyrefitte CN. Development of a TaqMan® RT-PCR assay without RNA extraction step for the detection and quantification of African Chikungunya viruses. Journal of Virological Methods. 2005 Mar;124(1–2):65–71.
3. Calvez E, Bounmany P, Somlor S, Xaybounsou T, Viengphouthong S, Keosenhom S, et al. Multiple chikungunya virus introductions in Lao PDR from 2014 to 2020. PLoS One. 2022;17(7):e0271439.
4. Lim SK, Lim JK, Yoon IK. An Update on Zika Virus in Asia. Infection & Chemotherapy. 2017 Jun 1;49(2):91– 100.
5. Calvez E, Vetsaphong P, Somlor S, Xaybounsou T, Viengphouthong S, Dupont-Rouzeyrol M, et al. First probable case of congenital Zika syndrome in Lao People’s Democratic Republic. Int J Infect Dis. 2021 Apr;105:595–7.







